Suppose you are a scientist studying the reactions of SO 2 and O 2 . If you
Question:
Suppose you are a scientist studying the reactions of SO2 and O2. If you wish to use gas molar concentrations, you must first convert the equilibrium constant K to Kc. At 400 8C, the equilibrium constant K for 2 SO2(g) + O2(g) ⇌ 2 SO3(g) is 3.1 * 104. What is the value of Kc at this temperature?
PLAN Because P° = 1 bar and c° = 1 mol · L–1, it is sensible to use R expressed in bar and liters, R = 8.3145 * 10–2 L · bar · K–1 · mol–1, and to note that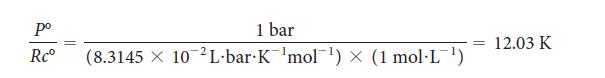
Then, Eq. 4a can be written as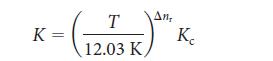
To use this equation, identify the value of Δnr for the reaction, convert the temperature to the Kelvin scale, and rearrange it to solve for Kc.
What should you assume? Assume that the gases are ideal.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





