The German physicist Lothar Meyer observed a periodicity in the physical properties of the elements at about
Question:
The German physicist Lothar Meyer observed a periodicity in the physical properties of the elements at about the same time that Mendeleev was working on their chemical properties. Some of Meyer’s observations can be reproduced by examining the molar volume for the solid element as a function of atomic number.
Calculate the molar volumes for the elements in Periods 2 and 3 from the densities of the elements found in Appendix 2D and the following solid densities (in g·cm-3): nitrogen, 0.88; fluorine, 1.11; neon, 1.21. Plot your results against atomic number and explain any variations that you observe.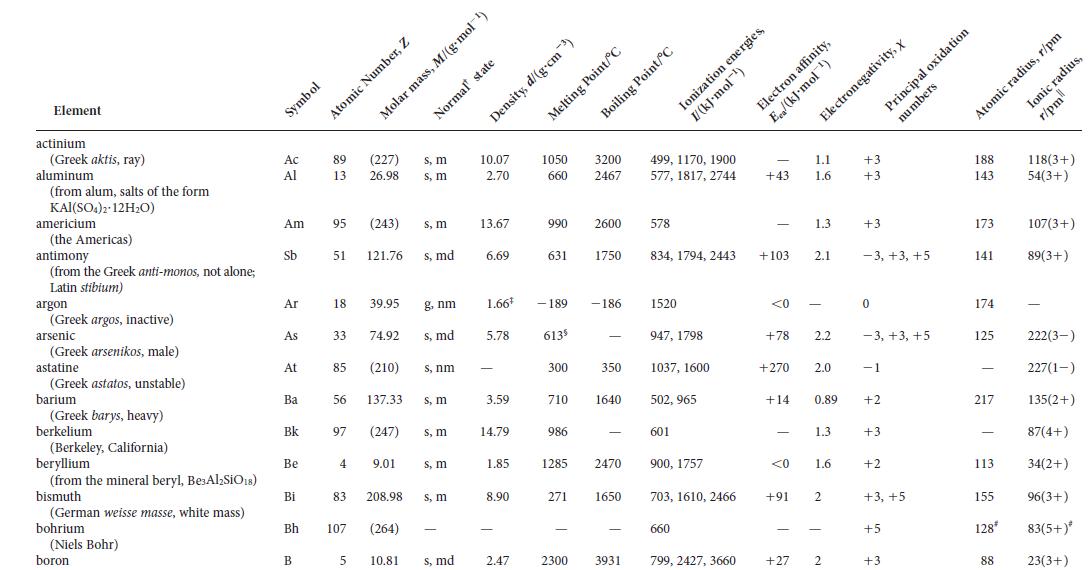
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





