The Lewis structure of caffeine, C 8 H 10 N 4 O 2 , a common stimulant,
Question:
The Lewis structure of caffeine, C8H10N4O2, a common stimulant, is given below.
(a) Give the hybridization of each atom other than hydrogen.
(b) On the basis of your answers in part (a), estimate the bond angles around each carbon and nitrogen atom.
(c) Search the chemical literature for the structure of caffeine, and compare the observed structural parameters with your predictions.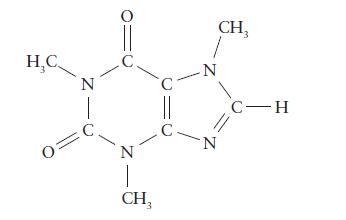
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





