The pre-equilibrium and the steady-state approximations are two different approaches to deriving a rate law from a
Question:
The pre-equilibrium and the steady-state approximations are two different approaches to deriving a rate law from a proposed mechanism. For the following mechanism, determine the rate law
(a) By the pre-equilibrium approximation and
(b) By the steady-state approximation.
(c) Under what conditions do the two methods give the same answer?
(d) What will the rate laws become at high concentrations of Br–?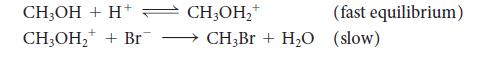
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





