The reaction [2 mathrm{I}^{-}(a q)+mathrm{S}_{2} mathrm{O}_{8}{ }^{2-}(a q) longrightarrow mathrm{I}_{2}(a q)+2 mathrm{SO}_{4}{ }^{2-}(a q)] was studied at
Question:
The reaction
\[2 \mathrm{I}^{-}(a q)+\mathrm{S}_{2} \mathrm{O}_{8}{ }^{2-}(a q) \longrightarrow \mathrm{I}_{2}(a q)+2 \mathrm{SO}_{4}{ }^{2-}(a q)\]
was studied at \(25^{\circ} \mathrm{C}\). The following results were obtained, where
\[\text { Rate }=-\frac{d\left[\mathrm{~S}_{2} \mathrm{O}_{8}{ }^{2-}ight]}{d t}\]
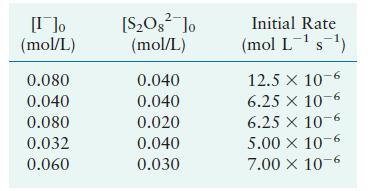
a. Determine the rate law.
b. Calculate the value of the rate constant for each experiment and an average value for the rate constant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





