Question: The unit cell for a pure xenon fluoride compound is shown in the following diagram. a. What is the formula of the compound? b. The
The unit cell for a pure xenon fluoride compound is shown in the following diagram.
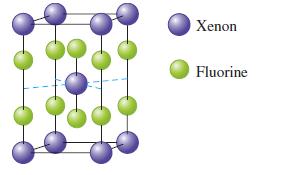
a. What is the formula of the compound?
b. The unit cell of \(\mathrm{XeF}_{2}\) has a height of \(702 \mathrm{pm}\), and the edge of the square base has a length of \(432 \mathrm{pm}\). Calculate the density of \(\mathrm{XeF}_{2}\).
Xenon Fluorine
Step by Step Solution
3.46 Rating (172 Votes )
There are 3 Steps involved in it
The arrangement of atoms in the unit cell must be understood in order to calculate the compounds for... View full answer

Get step-by-step solutions from verified subject matter experts


