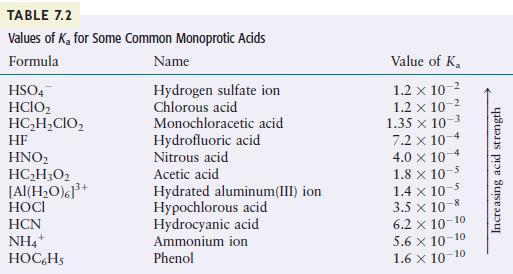
Use Table 7.2 to order the following from the strongest to the weakest acid. [mathrm{HClO}_{2}, mathrm{H}_{2} mathrm{O},
Question:
Use Table 7.2 to order the following from the strongest to the weakest acid.
\[\mathrm{HClO}_{2}, \mathrm{H}_{2} \mathrm{O}, \quad \mathrm{NH}_{4}^{+}, \quad \mathrm{HClO}_{4}\]
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10- 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10-5 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The strength of an acid is often determined by its acid dissociation constant Ka The larger the Ka t...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 7 18, reduce each rational expression to lowest terms. x 2 + 4x + 4 x2 x - 4
-
Use Table to order the following from the strongest to the weakest acid. HClO2, H2O, NH4+, HClO4 Table Formula Name Value of K 12 x 10-2 1.2 x 10-2 1.35 x 10-1 7.2 x 10-4 4.0 x 10-4 1.8 x 10-5 [ 1.4...
-
How do you identify the potential classes in a problem domain description?
-
With light falling normally on a transparent diffraction grating l0 mm wide, it was found that the components of the yellow line of sodium (589.0 and 589.6 rim) are resolved beginning with the fifth...
-
What is the role of the anchor MSC in GSM networks?
-
On April 20, 1992, Daniel Hubbard (plaintiff), a potato farmer, and UTZ Quality Foods, Inc. (UTZ) (defendant), a potato chip manufacturer, entered an installment contract under which Hubbard agreed...
-
1. Create an initial ERD for the new system that contains at least eight entities. 2. Analyze each relationship to determine if it is 1:1, 1:M, or M:N. 3. Normalize your designs for all tables to...
-
Calculate Inventory Conversion Period, Receivables Conversion Period, the Payment Conversion Period, and the Operating Cycle for Innovation Inc. given the following information. Use 365 to get an...
-
You may need Table 7.2 to answer the following questions. a. Which is the stronger acid, \(\mathrm{H}_{2} \mathrm{SO}_{4}\) or \(\mathrm{H}_{2} \mathrm{O}\) ? b. Which is the stronger acid,...
-
For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and the conjugate acid. a. \(\mathrm{H}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{CO}_{3} ightleftharpoons...
-
True or false: Despite years of intensive research, we still do not have even an outline of the mechanism that actually causes plants to begin producing flowers.
-
How many zongzi should she make to minimize the days customers are turned away and to adhere to a 10% target? Cost of ingredients and preparation of a single zongzi dish: INR 600 - Revenue from a...
-
A key aspect of creating a business or new product offering is finding a position in the market that will allow you to succeed. This allows you to offer to your target customers a product that has a...
-
The CEO of firm A emphasizes a wide span of control. The CEO of firm B emphasizes a narrow span of control. What is the outcome for the first CEO compared with the second?
-
1. What are some of the major goals of the budgeting process? Explain. 2. Explain conservative budgeting. Why should you practice conservative budgeting? 3. What does it mean to consider Macro &...
-
Lee and Julia are common-law partners, both aged 35. Lee's mother, aged 75, lives with them and is financially dependant on Lee. While applying for life insurance, Lee mentions that if he dies, he...
-
Omega Computers, Inc., is a franchisor that grants exclusive geographic territories to its franchisees with retail locations, including Petes Digital Products. After selling more than two hundred...
-
One Way Cellular accountants have assembled the following data for the year ended September 30, 2014: Prepare the operating activities section using the indirect method for One Way Cellulars...
-
Scientists often find it helpful to use very simple expressions to estimate the order of magnitude of a property without doing a detailed calculation. Treat a hydrogen atom as a one-dimensional box...
-
Arrange the elements in each of the following sets in order of decreasing atomic radius: (a) Sulfur, chlorine, silicon; (b) Cobalt, titanium, chromium; (c) Zinc, mercury, cadmium; (d) Antimony,...
-
Rainbows form when the wavelengths of sunlight are refracted (bent) through different angles. When sunlight passes through droplets of water, the shorter the wavelength of the light, the greater the...
-
Many companies debut their newest commercials during the Super Bowl. For some, the commercials have even become a bigger attraction than the game. Can either watch the game and make note of several...
-
If a consumer previously exhibited strong brand loyalty what is the most likely explanation for a customer who visited the hair salon after a gap of over 140 days since the previous visit. ?
-
In thinking about the great brand success Red Bull has experienced, what do they need to think about in the future with respect to communication strategies? Think about both traditional and digital...

Study smarter with the SolutionInn App


