Question: Use the information in Table 9C.1 to write the formula for each of the following coordination compounds: (a) Potassium hexacyanidochromate(III) (b) Pentaamminesulfatocobalt(III) chloride (c) Tetraamminediaquacobalt(III)
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds:
(a) Potassium hexacyanidochromate(III)
(b) Pentaamminesulfatocobalt(III) chloride
(c) Tetraamminediaquacobalt(III) bromide
(d) Sodium bisoxalato(diaqua)ferrate(III)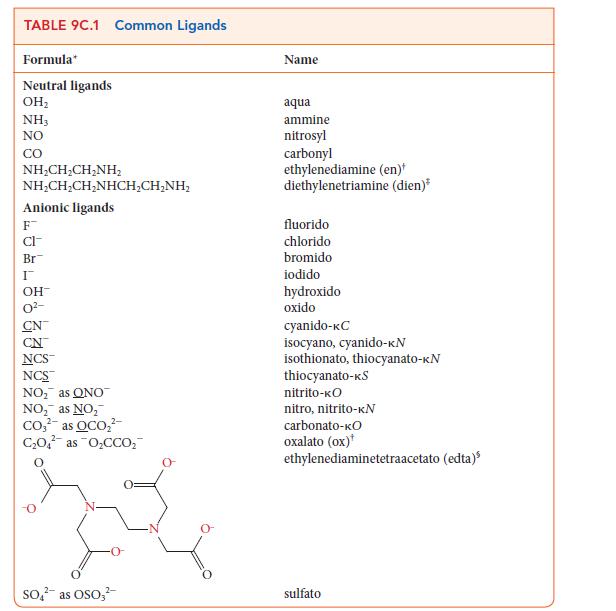
TABLE 9C.1 Common Ligands Formula Neutral ligands OH NH3 NO CO NHCH,CH_NH, NHCH,CH,NHCH,CHNH, Anionic ligands F CI- Br I OH- CN NCS NCS NO as ONO NO as NO Co as OCO CO4 as OCCO 2- SO as OSO3 Name aqua ammine nitrosyl carbonyl ethylenediamine (en)* diethylenetriamine (dien)* fluorido chlorido bromido iodido hydroxido oxido cyanido-KC isocyano, cyanido-KN isothionato, thiocyanato-KN thiocyanato-KS nitrito-KO nitro, nitrito-KN carbonato-KO oxalato (ox) ethylenediaminetetraacetato (edta) sulfato
Step by Step Solution
There are 3 Steps involved in it
a KaCrCN6 b ... View full answer

Get step-by-step solutions from verified subject matter experts


