Question: Using the bond energies listed in Table 13.6, calculate H for the reaction of methane with chlorine and fluorine to give Freon-12 (CF 2 Cl
Using the bond energies listed in Table 13.6, calculate ΔH for the reaction of methane with chlorine and fluorine to give Freon-12 (CF2Cl2).
![]()
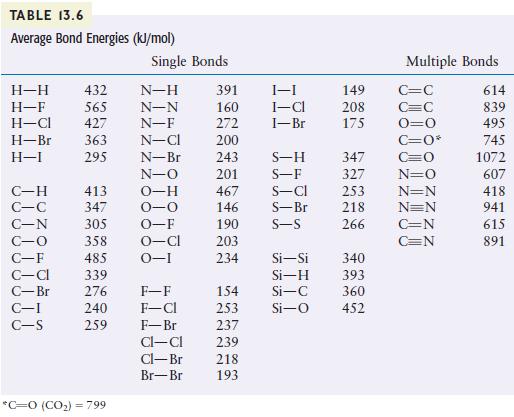
CH(g) + 2Cl(g) + 2F(g) CFCl(g) + 2HF(g) + 2HCl(g)
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
The idea here is to break the bonds in the reactants to give indi vidual atoms and then assemble the... View full answer

Get step-by-step solutions from verified subject matter experts


