When radioactive nuclides are used in medicine and industry, it is important to know what daughter nuclide
Question:
When radioactive nuclides are used in medicine and industry, it is important to know what daughter nuclide to expect, as that nuclide might also be radioactive. Suppose you are working in a medical nuclear research laboratory and are studying calcium-41 and oxygen-15. What nuclide is produced when
(a) Calcium-41 undergoes electron capture;
(b) Oxygen-15 undergoes positron emission?
ANTICIPATE (a) In electron capture, a proton is turned into a neutron and, although there is no change in mass number, the atomic number is reduced by 1 (FIG. 10A.9). (b) A positron has the same tiny mass as an electron but a single positive charge. Positron emission can be thought of as the positive charge emitted by a proton as it is converted into a neutron. As a result, the atomic number decreases by 1 but there is no change in mass number (FIG. 10A.10).
PLAN Write the nuclear equation for each reaction, representing the daughter nuclide as E, with atomic number Z and mass number A. Then find Z and A from the requirement that both mass number and atomic number are conserved in a nuclear reaction.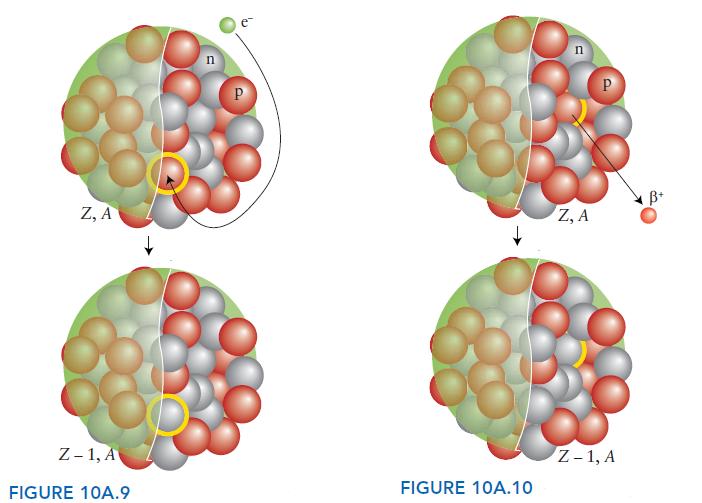
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





