When the rate of the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g)
Question:
When the rate of the reaction 2 NO(g) + O2(g) → 2 NO2(g) was studied, the rate was found to double when the O2 concentration alone was doubled but to quadruple when the NO concentration alone was doubled. Which of the following mechanisms accounts for these observations? Explain your reasoning.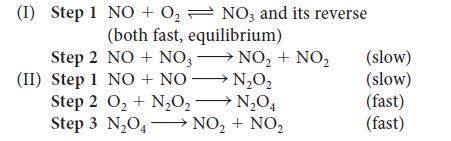
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





