Which substance in each of the following pairs would you expect to have the higher standard molar
Question:
Which substance in each of the following pairs would you expect to have the higher standard molar entropy at 298 K and 1 bar? Explain your reasoning.
(a) Lodine vapor or bromine vapor;
(b) The two liquids cyclopentane and 1-pentene (see structures);
(c) Ethene (ethylene) or an equivalent mass of polyethylene, a substance formed by the polymerization of ethylene.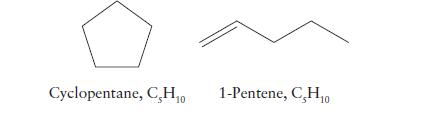
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





