Write the equilibrium constant for the reaction HIO 3 (aq) + NH 2 NH 2 (aq)
Question:
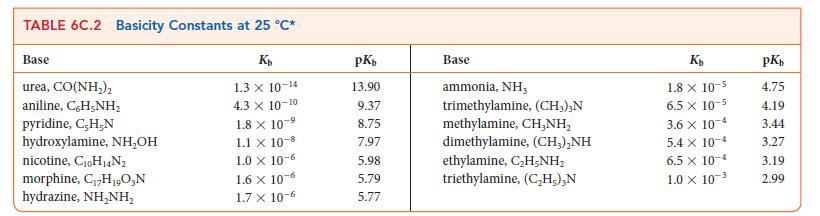
Write the equilibrium constant for the reaction HIO3(aq) + NH2NH2(aq) ⇌ NH2NH3 + (aq) + IO3– (aq) and calculate the value of K at 298 K using the data in Tables 6C.1 and 6C.2.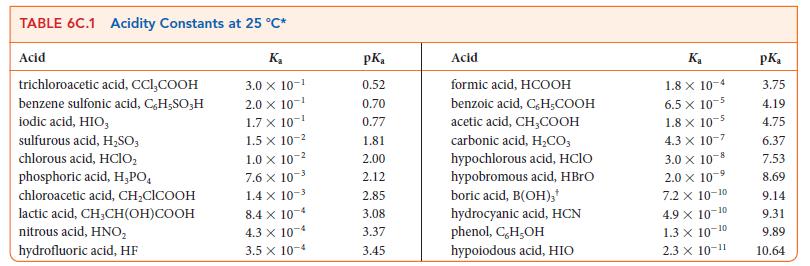 .
.
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 C* Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H-SO;H iodic acid, HIO, sulfurous acid, HSO3 chlorous acid, HClO phosphoric acid, H,PO chloroacetic acid, CHClCOOH lactic acid, CH,CH(OH)COOH nitrous acid, HNO hydrofluoric acid, HF K 3.0 X 10- 2.0 10- 1.7 X 10- 1.5 x 10- 1.0 x 10- 7.6 X 10- 1.4 x 10- 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H-COOH acetic acid, CHCOOH carbonic acid, HCO; hypochlorous acid, HClO hypobromous acid, HBrO boric acid, B(OH)3* hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO Ka 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 10-8 2.0 x 10-5 -9 7.2 x 10-10 4.9 X 10- -10 1.3 10-10 2.3 10-11 pK 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
K NHNH IO KHIOxK NHNH N...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Estimate the slope of the tangent line at the point indicated. y(x) = 1 x + 2 x = 2
-
Find the mass of the following thin bars with the given density function. p(x) = [x if 0 x 1 x(2-x) if 1 < x 2
-
Assume that a security is selling at INR 217 and American call and American put options are available on the stock with 3 months maturity and an exercise price of INR 210. The call is selling at INR...
-
Accounting for a byproduct. Washington Oceanic Water (WOW) desalinates and bottles sea water. The desalinated water is in high demand from a large group of environmentally conscious people on the...
-
What are advantages of having architectural standards?
-
What are the three main objectives of any questionnaire?
-
On June 1, Fab Salad Dressings creates a petty cash fund with an imprest balance of $300. During June, Al Franklin, the fund custodian, signs the following petty cash tickets: On June 30, prior to...
-
Can you elaborate on the iterative nature of your strategic planning process, highlighting mechanisms for continuous learning, adaptation, and refinement in response to evolving environmental...
-
A chemist attempts to separate barium ions from lead ions by using the sulfate ion as a precipitating agent. (a) What sulfate ion concentrations are required for the precipitation of BaSO 4 and PbSO...
-
Decide whether an aqueous solution of each of the following salts has a pH equal to, greater than, or less than 7. If pH > 7 or pH < 7, write a chemical equation to justify your answer. (a) NH 4 Br;...
-
In problem, (a) Graph each quadratic function by determining whether its graph opens up or down and by finding its vertex, axis of symmetry, y-intercept, and x-intercepts, if any. (b) Determine the...
-
What relevant information is provided in each capital budgeting method?
-
For the funding of projects, why are municipalities moving from the use of general obligation bonds to revenue bonds, PFA bonds, and certificates of participation?
-
What major information (data) do you need for capital budgeting when you want to compare projects?
-
Explain why a relegation system would be difficult to implement in North American professional sport leagues.
-
How can a stadium or arena be built without putting too much financial burden on a local government?
-
Would the issue in the above question become moot if we employed predetermined overhead rates? Why does the answer depend on whether the decline in volume was predictable?
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
Within what range can you restrict the values of P and/or T if the following information is known about sulfur? Use Figure 8.11 to answer this problem. a. Only the rhombic solid phase is observed for...
-
The normal melting point of H 2 O is 273.15 K, and H fusion = 6010 J mol -1 . Calculate the change in the normal freezing point at 100. and 500. bar compared to that at 1 bar assuming that the...
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
Identify the two key functions of management: leading, controlling, and accurately describing specific activities under each. Compare and contrast provide two examples each for your critical thinking...
-
Consider the following abstract algorithm. for i = 1 to n Call method MyMethod(); endfor Assume that the method MyMethod() runs in O() time. Express the algorithm's runtime using Big-Oh notation;
-
Jeff Johanson is a proprietor who owns a small retail shop that sells clothing. The company pays $7 for a shirt and later sells it for $24. The company will recognize $17 of ?

Study smarter with the SolutionInn App


