A chemical reaction A B is carried out in a closed vessel. The following data are
Question:
A chemical reaction A → B is carried out in a closed vessel. The following data are taken for the concentration of A, CA(g/L), as a function of time, t(min), from the start of the reaction:
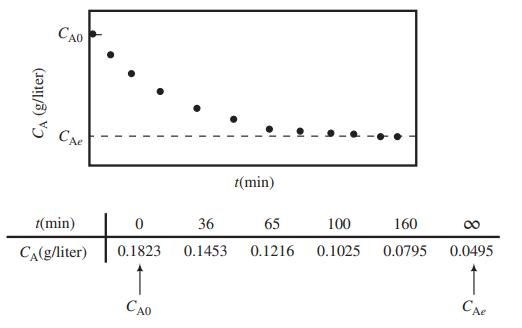
A proposed reaction mechanism predicts that CA and t should be related by the expression
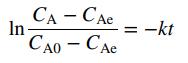
where k is the reaction rate constant.
(a) Do the data support this prediction? If so, plot the data appropriately to determine the value of k.
(b) If the tank volume is 125 L and there is no B in the tank at t = 0, how much B(g) does the tank contain after two hours?
(c) Estimate the time required for the final concentration of A to reach 1.1, 1.05, and 1.01 times CAe, and determine the mass of B produced at each of these conditions for the reactor described in Part (b).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





