A 1.00-L mixture of helium, neon, and argon has a total pressure of 662 mmHg at 298
Question:
A 1.00-L mixture of helium, neon, and argon has a total pressure of 662 mmHg at 298 K. If the partial pressure of helium is 341 mmHg and the partial pressure of neon is 112 mmHg, what mass of argon is present in the mixture?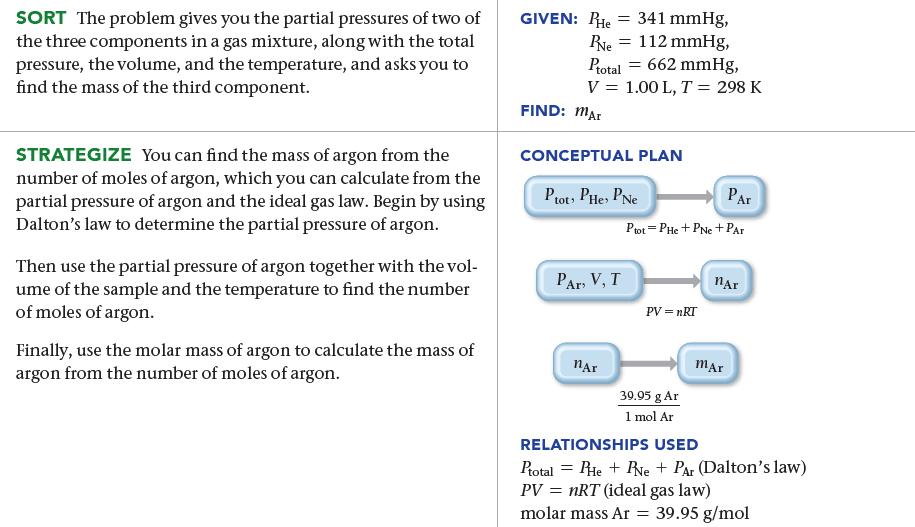
Transcribed Image Text:
SORT The problem gives you the partial pressures of two of the three components in a gas mixture, along with the total pressure, the volume, and the temperature, and asks you to find the mass of the third component. STRATEGIZE You can find the mass of argon from the number of moles of argon, which you can calculate from the partial pressure of argon and the ideal gas law. Begin by using Dalton's law to determine the partial pressure of argon. Then use the partial pressure of argon together with the vol- ume of the sample and the temperature to find the number of moles of argon. Finally, use the molar mass of argon to calculate the mass of argon from the number of moles of argon. GIVEN: PHE = 341 mmHg, Re = 112 mmHg, Ptotal = 662 mmHg, V = 1.00 L, T = 298 K FIND: MAT CONCEPTUAL PLAN Plot PHe, PNe PAT, V, T nAr PAR Ptot - PHe + PNE + PAT PV = nRT 39.95 g Ar 1 mol Ar nAr mar RELATIONSHIPS USED Ptotal = He + Ne + Par (Dalton's law) PV = nRT (ideal gas law) molar mass Ar = 39.95 g/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Ptotal PHe RNe PAT PAr Ptotal PH PNe 662 mmHg 341 mmHg 1...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 1.50-L mixture of helium, neon, and argon has a total pressure of 754 mmHg at 310 K. If the partial pressure of helium is 431 mmHg and partial pressure of neon is 211 mmHg, what mass of argon is...
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
Consider the reaction 2 NO2(g) N2O4(g). (a) Using data from Appendix C, calculate G at 298 K. (b) Calculate G at 298 K if the partial pressures of NO2 and N2O4 are 0.40 atm and 1.60 atm,...
-
Explain the nature of stress at work Describe the health consequences of stressful work Explain how to use hardiness theory to reduce stress List three ways to use Banduras self-efficacy theory to...
-
What are blogs? How are they used? Who is using them?
-
The following information is taken from the accounts of Latta Company. The entries in the T-accounts are summaries of the transactions that affected those accounts during the year. The overhead that...
-
Consider the following cash flow profile, and assume MARR is 10 percent/year and the finance rate is 4 percent/year. a. Determine the MIRR for this project. b. Is this project economically...
-
On November 1, 2013, Norwood borrows $ 200,000 cash from a bank by signing a five-year installment note bearing 8% interest. The note requires equal total payments each year on October 31. Required...
-
Research Web-based database technologies and identify a database management system (other than SQL Server, MySQL, or Oracle) that is used to deploy applications to the Web and the cloud. Discuss the...
-
Why is it impossible to breathe air through an extra-long snorkel (longer than a couple of meters) while swimming under water?
-
A gas sample at STP contains 1.15 g oxygen and 1.55 g nitrogen. What is the volume of the gas sample? a) 1.26 L b) 2.04 L c) 4.08 L d) 61.0 L
-
The following situations involve the application of the time value of money concept: a. Jan Cain deposited $19,500 in the bank on January 1, 1999, at an interest rate of 12% com-pounded annually. How...
-
Discuss the concept of cross-referencing.
-
What role do network providers play in the Internet commerce environment?
-
How does an enterprise system support the order-to-cash process?
-
Describe the methods used to integrate ERP systems with third-party modules, back-end or legacy systems, the Web, and business partners.
-
A convenience store? asked Stan, incredulous. Yep, a convenience store, replied Carrie Zabrinsky, or, as they say in the business, a c-store. Stan had arranged a meeting with his Subway development...
-
Jennifer Company purchases equipment by issuing a 7-year, $350,000 non-interest-bearing note, when the market rate for this type of note is 10%. Jennifer will pay off the note by an equal amount at...
-
r = 0.18 Find the coefficients of determination and non-determination and explain the meaning of each.
-
Determine the moments at B and C, then draw the moment diagram for the beam. Assume the supports at B and C are rollers and A and D are pins. EI is constant. 12 kN/m 12 kN/m A 4 m 6 m 4 m
-
Determine the reactions at the supports. Assume A is fixed and B and C are rollers that can either push or pull on the beam. EI is constant. 12 kN/m B. -2.5 m 5 m
-
Determine the moments at B and C, then draw the moment diagram for the beam. All connections are pins. Assume the horizontal reactions are zero. EI is constant. 12 kN/m 4 m- 12 kN/m D. -4 m 4 m-
-
4. (a) What is internal rate of return (IRR). Find the IRR of Projects M and P. (Show your workings clearly by employing the trial-and-error method) (b) According to IRR, which project or projects...
-
Job A, currently in the backlog at Work Center 649, has an overall due date of 101 hours from now. Job A requires 25 hours of processing at Work Center 649. It also has downstream requirements (i.e.,...
-
You are a WHS officer at NMT Workforce. You have moved to a new branch, which has not yet implemented processes relating to the election of Health and Safety Representatives. You will use the NMT...

Study smarter with the SolutionInn App


