Question: A hydrochloric acid solution will neutralize a sodium hydroxide solution. Look at the molecular views showing one beaker of HCl and four beakers of NaOH.
A hydrochloric acid solution will neutralize a sodium hydroxide solution. Look at the molecular views showing one beaker of HCl and four beakers of NaOH. Which NaOH beaker will just neutralize the HCl beaker? Begin by writing a balanced chemical equation for the neutralization reaction.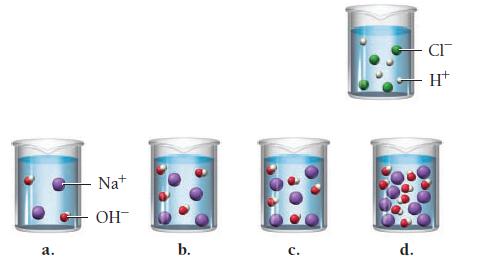
a. Na+ OH b. C. d. CI H+
Step by Step Solution
★★★★★
3.41 Rating (145 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


