A meteor contains 0.556 g of Pb-206 to every 1.00 g of U-238. Assuming that the meteor
Question:
A meteor contains 0.556 g of Pb-206 to every 1.00 g of U-238. Assuming that the meteor did not contain any Pb-206 at the time of its formation, determine the age of the meteor. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years.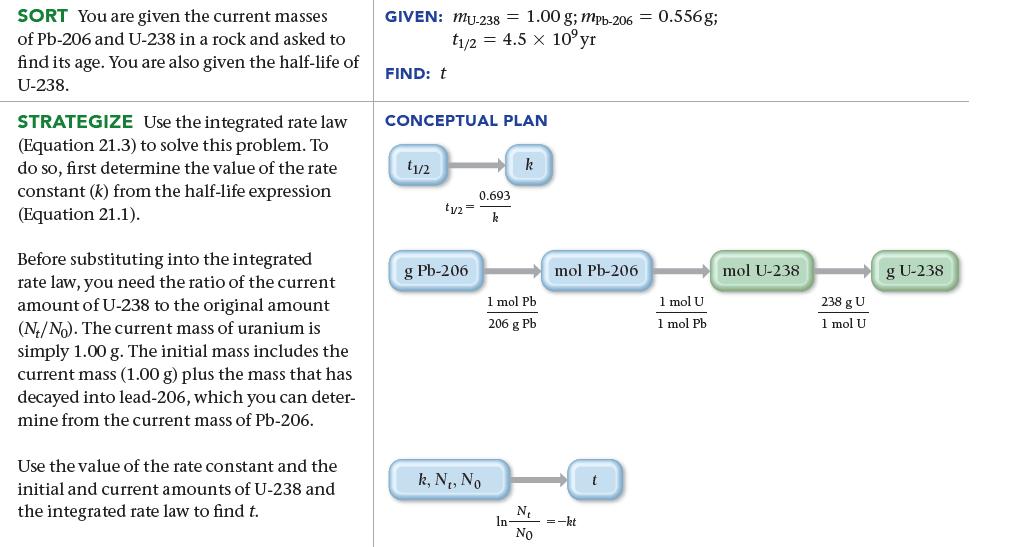
Transcribed Image Text:
SORT You are given the current masses of Pb-206 and U-238 in a rock and asked to find its age. You are also given the half-life of U-238. STRATEGIZE Use the integrated rate law (Equation 21.3) to solve this problem. To do so, first determine the value of the rate constant (k) from the half-life expression (Equation 21.1). Before substituting into the integrated rate law, you need the ratio of the current amount of U-238 to the original amount (N/No). The current mass of uranium is simply 1.00 g. The initial mass includes the current mass (1.00 g) plus the mass that has decayed into lead-206, which you can deter- mine from the current mass of Pb-206. Use the value of the rate constant and the initial and current amounts of U-238 and the integrated rate law to find t. GIVEN: MU-238 = 1.00 g; mpb-206 = 0.556g; t1/2 = 4.5 x 10°yr FIND: t CONCEPTUAL PLAN 11/2 tu= g Pb-206 0.693 k k, N₁, No k 1 mol Pb 206 g Pb In- N₁ No mol Pb-206 =-kt t 1 mol U 1 mol Pb. mol U-238 238 g U 1 mol U g U-238
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
t12 0693 k 0693 t12 154 x 100yr k 5 Nt In No ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The contents of the rock have a 206Pb to 238U mass ratio of 0.175:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Sketch the following regions and write an iterated integral of a continuous function f over the region. Use the order dy dx. R = {(x, y): 0 x 4, x y 8x}
-
Web Design Services billed its customers a total of $490,200 for the month of August, including 9 percent federal excise tax and 5 percent sales tax. 1. Determine the proper amount of service revenue...
-
Irresistible Art produces collectible pieces of art. The company's Raw Material Inventory account includes the costs of both direct and indirect materials. Account balances for the company at the...
-
Petitioner Salman was indicted for federal securities-fraud crimes for trading on inside information he received from a friend and relative-by-marriage, Michael Kara, who, in turn, had received the...
-
BAK Corp. is considering purchasing one of two new diagnostic machines. Either machine would make it possible for the company to bid on jobs that it currently isnt equipped to do. Estimates regarding...
-
Let us consider a two-link mechanism shown in figure below. Assume that 91=0 and 92 = 45; a = a = 1; -(x,y) a. Determine the Jacobian matrix for this configuration. b. Calculate the Yoshikawa's...
-
Consider this graph representing the decay of a radioactive nuclide. What is the half-life of the nuclide? (a) 625 years (b) 1250 years (c) 2500 years (d) 3125 years
-
The chart shows the mass of a decaying nuclide versus time. What is the half-life of the decay? a) 15 min b) 25 min c) 35 min d) 70 min Mass Nuclide (g) 30- 25 DO 20- 15 10 5 0 0 20 40 60 80 Time...
-
What does DASD stand for? a. Database appropriate storage device. b. Directly accumulative storage device. c. Directly accessible storage device. d. Data accumulative storage device.
-
20. Draw the output F for the pattern of inputs shown in the figure below for a two input AND gate. x AND y F F= x y X 0 0 1 1 FL 0 1 0 1
-
Marcel Co. is growing quickly. Dividends are expected to grow at a rate of 0.06 for the next 4 years, with the growth rate falling off to a constant 0.04 thereafter. If the required return is 0.07...
-
A 3.125 percent TIPS has an original reference CPI of 185.7. If the current CPI is 211.0, what is the par value and current interest payment of the TIPS? (Do not round intermediate calculations....
-
An error exists in a set of financial statements. The error is not pervasive, but material. The CPA can issue what type of report? Explain.
-
Caspian Sea Drinks is considering buying the J-Mix 2000. It will allow them to make and sell more product. The machine cost $1.22 million and create incremental cash flows of $557,216.00 each year...
-
After completing its capital spending for the year, Carlson Manufacturing has $1,000 extra cash. Carlson's managers must choose between investing the cash in Treasury bonds that yield 8 percent or...
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
A thin plastic rod 20 cm long carries 3.2 nC distributed uniformly over its length. (a) If the rod is bent into a ring, find the potential at its center. (b) If its bent into a semicircle, find the...
-
A thin ring of radius R carries charge 3Q distributed uniformly over three-fourths of its circumference, and -Q over the rest. Find the potential at the rings center.
-
The potential at the center of a uniformly charged ring is 45 kV, and 15 cm along the ring axis the potential is 33 kV. Find the rings radius and total charge.
-
The Naruto Corporation had the following historical MCIT and RCIT data: 2018 2019 2020 2021 MCIT P 120,000 P 200,000 P 190,000 P 170,000 RCIT P 110,000 P 220,000 P 0 180,000 In the immediately...
-
Patrice recently returned to Australia after working in Singapore for seven years. On 1 January she gained her Australian tax residency. Her retirement savings accumulated in a Singaporean fund at...
-
Albright Inc. has recently issued a 10% stock dividend to its existing stockholders. As a result of the issuance of the stock dividend the market price of the stock declined 25%. Albright has...

Study smarter with the SolutionInn App


