A solution is 0.0250 M in Pb 2+ . What minimum concentration of Cl is required
Question:
A solution is 0.0250 M in Pb2+. What minimum concentration of Cl– is required to begin to precipitate PbCl2?
For PbCl2, Ksp = 1.17 * 10-5.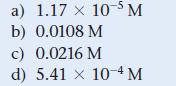
Transcribed Image Text:
a) 1.17 x 10-5 M b) 0.0108 M c) 0.0216 M d) 5.41 x 10-4 M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
c...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the Cl concentration just as Ag2CrO4 begins to precipitate when 1.0 MAgNO3 is slowly added to a solution containing 0.015 M Cl and 0.015 M CrO42?
-
Discuss how laws and regulations guide total compensation. Research a law or regulation and discuss how it influences total compensation in your organization or an organization you are familiar with....
-
A solution of Na2SO4 is added drop wise to a solution that is 0.010 M in Ba2+ and 0.010 M in Sr2+. (a) What concentration of SO42- is necessary to begin precipitation? (Neglect volume changes. BaSO4...
-
Find the point on the plane z = x + y + 1 closest to the point P = (1, 0, 0). Minimize the square of the distance.
-
Gator Corporation manufactures several types of accessories. For the year, the gloves and mittens line had sales of $500,000, variable expenses of $370,000, and fixed expenses of $150,000. Therefore,...
-
Baker Brothers has a DSO of 40 days, and its annual sales are $7,300,000. What is its accounts receivable balance? Assume that it uses a 365-day year.
-
Beng-Yu Woo, Xiaoming Li, and Vivian Hsiun created and patented an invention titled Full Duplex Single Chip Video Codec. At the time, Woo, Li, and Hsiun were employees of Infochips Systems, Inc....
-
Hallas Company manufactures a fast-bonding glue in its Northwest plant. The company normally produces and sells 40,000 gallons of the glue each month. This glue, which is known as MJ-7, is used in...
-
A jewelry shop specializes in creating gold and silver charms. The shop has five metalsmiths who work on the charms. One week, the shop's production possibilities curve shifts inward. What could...
-
Which compound is more soluble in an acidic solution than in a neutral solution? a) PbBr 2 b) CuCl c) AgI d) BaF 2
-
You add potassium hydroxide to the solution in Example 18.13. When the [OH - ] reaches 1.9 * 10 -6 M (as you just calculated), magnesium hydroxide begins to precipitate out of solution. As you...
-
What is the relevance of the classification of levels of activity to ABC?
-
Which factors determine cost structure?
-
What types of revenues of households can be distinguished?
-
What are the different goal types of economic entities?
-
What is the level of activity?
-
Many college students pull all-nighters to study for midterms and finals. What Andrew Kozlovski noticed when he was a freshman at the University of Southern California was how many students were...
-
We offer a guitar at every price point for every skill level, explains Kevin Lello, vice president of marketing at Washburn Guitars. Washburn is one of the most prestigious guitar manufacturers in...
-
(a) What is the focal length of a magnifying glass that gives an angular magnification of 8.0 when the image is at infinity? (b) How far must the object be from the lens?
-
Obtain the Laplace transform of f (t) in Fig. 15.28 . f(t) A 15 1 2 3 4 t
-
Find the Laplace transform of the signal in Fig. 15.26. f(t) A 25 2 4
-
Determine the Laplace transform of 3.5 cos (5t 45).
-
Aces Incorporated, a manufacturer of tennis rackets, began operations this year. The company produced 6,100 rackets and sold 5,000. Each racket was sold at a price of $91. Fixed overhead costs are...
-
Blossom Company purchased a depreciable asset for $174000. The estimated salvage value is $13000, and the estimated useful life is 10 years. The straight-line method will be used for depreciation....
-
Terry was ill for three months and missed work during this period. During the illness, Terry received $5,450 in sick pay from a disability insurance policy. What amounts are included in Terry's gross...

Study smarter with the SolutionInn App


