An LP gas tank in a home barbeque contains 13.2 kg of propane, C 3 H 8
Question:
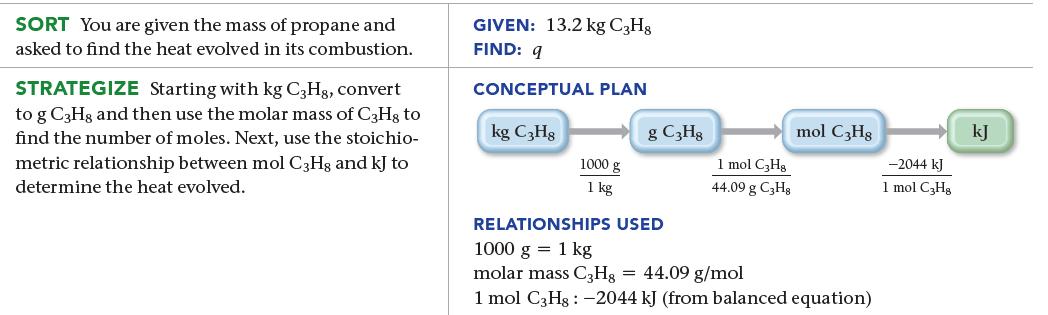
An LP gas tank in a home barbeque contains 13.2 kg of propane, C3H8. Calculate the heat (in kJ) associated with the complete combustion of all of the propane in the tank.![]()
Transcribed Image Text:
C3Hg(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) ΔΗχη = –2044 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
1000 g 132 kg C3...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A propane tank on a home barbeque contains 10.4 * 10 3 g of propane. a. Write the balanced chemical reaction for the combustion of gaseous propane (C 3 H 8 ) to form water vapor and gaseous carbon...
-
A gas tank in a Toyota Corolla is 13.2 gallons in capacity. How many liters of gasoline when full will this tank hold. Round to nearest hundredth.
-
A rigid tank contains an ideal gas at 40°C that is being stirred by a paddle wheel. The paddle wheel does 200 kJ of work on the ideal gas. It is observed that the temperature of the ideal gas...
-
As we continue our discussions regarding revenue's associated with public sector, this will help reinforce some of the ideas regarding taxes on goods and services. Through the next week, save your...
-
Diewold Company has two departments, Milling and Assembly. The company uses a job-order cost system and computes a predetermined overhead rate in each department. The Milling Department bases its...
-
Darlene is a stunt motorcyclist in a traveling circus. For the climax of her show, she takes off from the ramp at angle , clears a fiery ditch of width x, and lands on an elevated platform (height H)...
-
A U.S. Treasury bond pays a 7% coupon on January 7 and July 7. How much interest accrues per \($100\) of principal to the bond holder between July 7, 2013, and August 9, 2013? How would your answer...
-
KinderKids provides daycare for children Mondays through Fridays. Its monthly variable costs per child are as follows: Lunch and snacks ................. $ 100 Educational supplies ...................
-
To determine the kinetics (rates) of ozone depletion reactions, chemists perform controlled laboratory studies. In this simulated lab, we will interpret data obtained from such laboratory experiments...
-
Consider the following thermochemical equation: What is the heat associated with the reaction of 6 moles of A? (a) -51.0 J (b) -306 J (c) -153 J (d) 153 J 2A AA 51.0J
-
A friend claims to have constructed a machine that creates electricity but requires no energy input. Explain why you should be suspicious of your friends claim.
-
Researchers dialogued with individual students on campus, but subsequent group interviews proved fruitless. Revise the following sentences to use plain language and familiar words.
-
What items are considered property?
-
In verifying the existence (and completeness) assertions, the auditor has the choice of which three audit strategies?
-
What are two control procedures over cash?
-
What are four controls that should be in place over inventory?
-
What items are included in cash balances?
-
Lavender Corporation had 10,000 shares of common stock outstanding at the beginning of the year. On July 1, it issued 5,000 shares, and on September 1 it reacquired 600 shares as treasury stock. What...
-
What is a content filter? Where is it placed in the network to gain the best result for the organization?
-
The prestressed concrete girder is made from plain stone concrete and four -in. cold-form steel reinforcing rods. Determine the dead weight of the girder per foot of its length. 8 in. 6 in. 20 in. 6...
-
The wall is 2.5 m high and consists of 51 mm à 102 mm studs plastered on one side. On the other side is 13 mm fiberboard, and 102 mm clay brick. Determine the average load in kN/m of length of...
-
A building wall consists of exterior stud walls with brick veneer and 13 mm fiberboard on one side. If the wall is 4 m high, determine the load in kN/m that it exerts on the floor.
-
1. Write an e-mail to your friend or family member who is doing wrong things with his or her life because he or she believes in some health myth. 2. Try to convince your friend or relative that...
-
What insights can be gleaned from research on achievement motivation regarding the interplay between achievement goals, self-regulation strategies, and academic or occupational outcomes ?
-
What would a Utilitarian do in this situation? Why? A woman's father suddenly dies of a heart attack. Shortly before his death, he asks his daughter, who is also a lawyer, to change his will to...

Study smarter with the SolutionInn App


