Borax, sodium tetraborate decahydrate, is an important mineral found in dry lakebeds in California. It is used
Question:
Borax, sodium tetraborate decahydrate, is an important mineral found in dry lakebeds in California. It is used to make soap and glass, and it is also used as a preservative. You can use the values of Ksp of borax at different temperatures to determine ΔH°, ΔS°, and ΔG° for the dissolution of borax: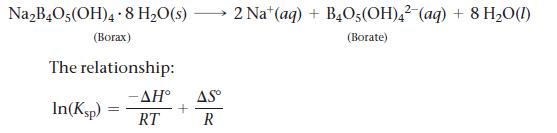
has the form of a linear equation y = mx + b, where y is the ln Ksp and x is 1/T. The slope is equal to (-ΔH°/R) and the y-intercept is ΔS°/R, where R is the gas constant, 8.314 J /K mol.
If you measure Ksp at several different temperatures, you can plot the lnK versus 1/T (T in Kelvin), as shown here.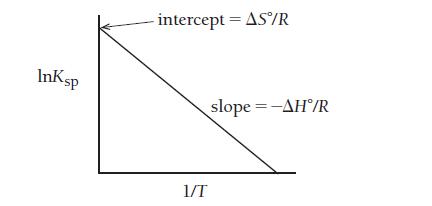
Knowing the values of ΔH° and ΔS° at a specific temperature allows the calculation of the change in Gibbs free energy for the reaction: ΔG° = ΔH° - T ΔS°.
The following table lists Ksp values for the dissolution of borax at several different temperatures (°C).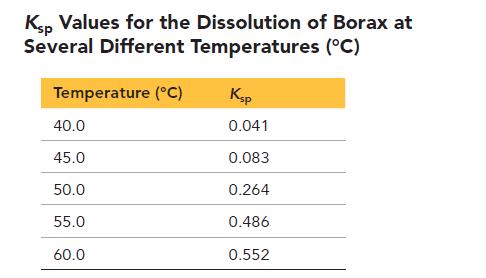
a. Plot a graph of lnKsp versus 1/T (T in Kelvin) and find the best-fitting line.
b. Determine ΔH°. Is this process endothermic or exothermic?
c. Determine ΔS°.
d. Determine ΔG°.
e. Sketch a graph of lnK versus 1/T for an exothermic process.
Step by Step Answer:






