Calculate G and K for each reaction the group created in Question 143. For one of the
Question:
Calculate ΔG° and K for each reaction the group created in Question 143. For one of the reactions, explain how the sign or magnitude of each quantity (E°cell, ΔG°, and K) is consistent with the fact that the reaction is spontaneous in the direction written.
Question 143
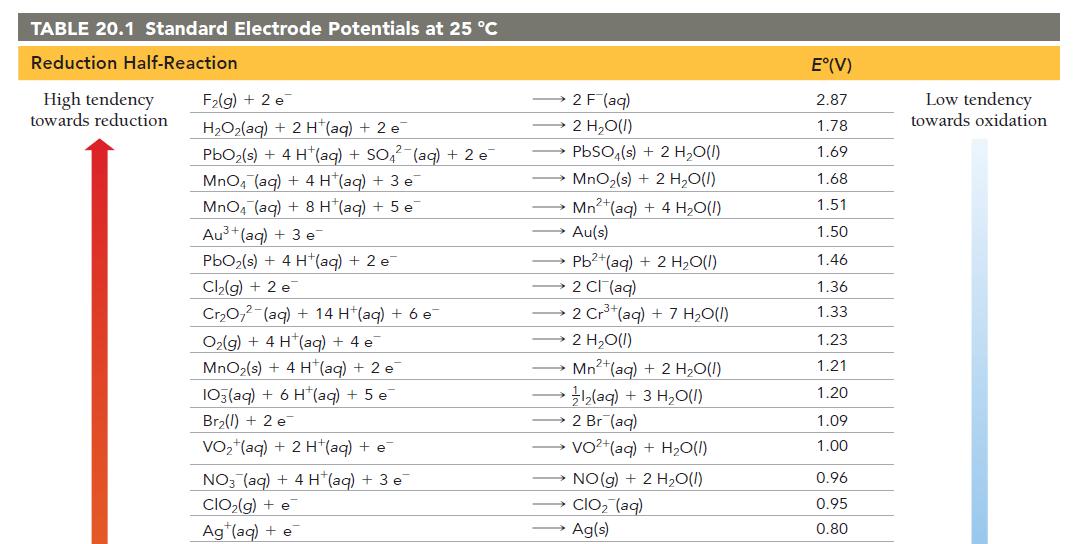
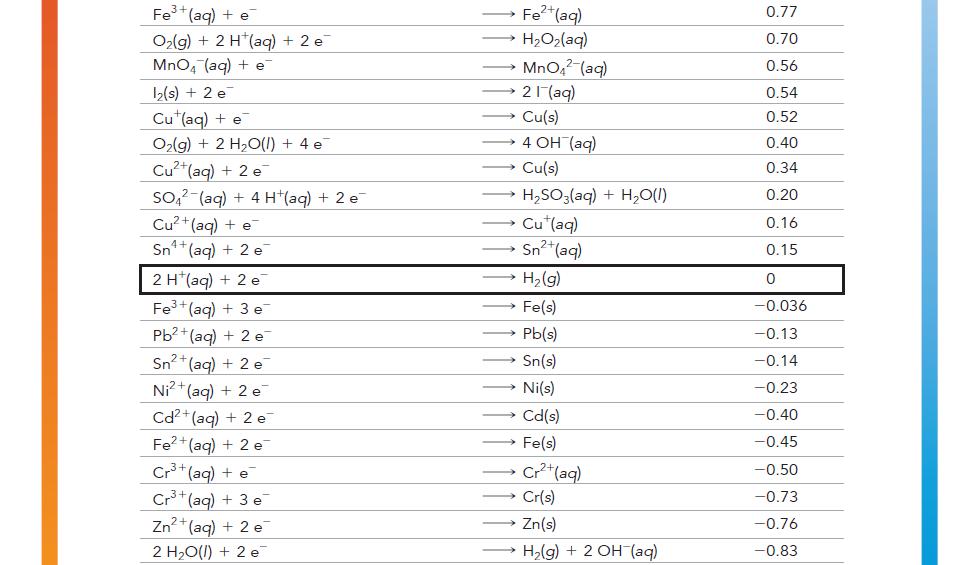
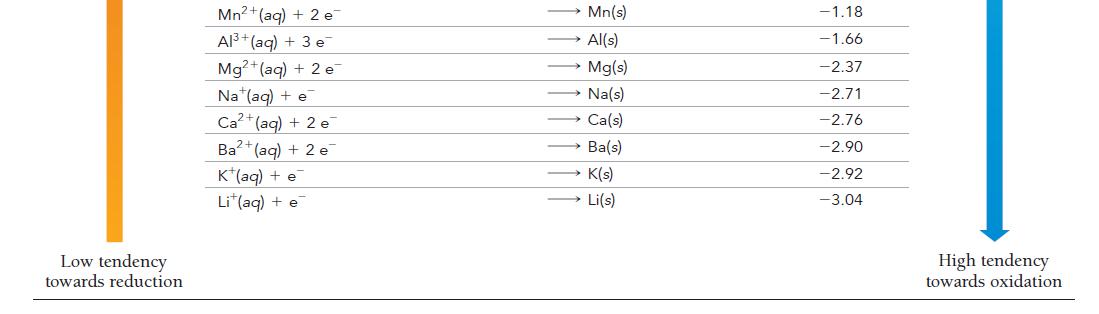
Have each group member select a half-reaction from Table 20.1.
Each member should calculate the standard cell potential of an electrochemical cell formed between each member’s halfreaction and the half-reaction of each of the other group members.
For each pair of half-reactions, write the overall balanced chemical reaction that will be spontaneous.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





