Calculate the equilibrium constant for each of the reactions in Problem 66. Problem 66 Use tabulated electrode
Question:
Calculate the equilibrium constant for each of the reactions in Problem 66.
Problem 66
Use tabulated electrode potentials to calculate ΔG°rxn for each reaction at 25 °C.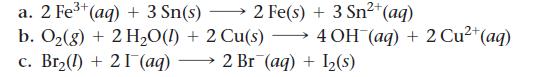
Transcribed Image Text:
2+ a. 2 Fe³+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn²+ (aq) b. O₂(g) + 2 H₂O(l) + 2 Cu(s) →→→ 4 OH(aq) + 2 Cu²+ (aq) c. Br₂(1) + 21 (aq) 2 Br (aq) + 1₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The equilibrium constant K for a chemical reaction is related to the standard free energy change Grxn for the reaction by the following equation Grxn ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant for each of the reactions in Problem 65. Problem 65 Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2...
-
Calculate the equilibrium constant for each of the reactions at25 ?C. Standard Electrode Potentials at 25 ?C Reduction Half-Reaction E ?(V) Pb2+( a q )+2 e ? ?Pb( s ) -0.13 Mg2+( a q )+2 e ? ?Mg( s )...
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. 2+ a. 2 Fe+ (aq) + 3 Sn(s) 2 Fe(s) + 3 Sn+ (aq) b. O(g) + 2 HO(l) + 2 Cu(s) 4 OH(aq) + 2 Cu+ (aq) c. Br(1) + 21 (aq)...
-
A financial institution can borrow $100 million for 2 years at 3%. It plans to invest this money in a 1-year security with an interest rate of 4.8% per year. Calculate net interest income for the...
-
Home Products Company manufactures a complete line of kitchen glassware. The Beverage Division specializes in 12-ounce drinking glasses. Erin Fisher, the superintendent of the Beverage Division,...
-
Thompson and Company plans to invest $8 million in a new product line. It expects the product to sell for $20 per unit. Variable costs are $8 per unit, and annual fixed operating costs (excluding...
-
The Buffalo Insurance Agency received the following notes during 2010: Requirements 1. Identifying each note by number, compute interest using a 360-day year, and determine the due date and maturity...
-
A plant asset with a cost of $40,000 and accumulated depreciation of $36,000 is sold for $6,000. Required a. What is the book value of the asset at the time of sale? b. What is the amount of gain or...
-
How do global leadership competencies differ from those required in domestic contexts, and what strategies can multinational organizations employ to develop and cultivate effective global leaders...
-
Calculate the equilibrium constant for the reaction between Ni 2+ (aq) and Cd(s) (at 25 C)
-
Use tabulated electrode potentials to calculate G rxn for each reaction at 25 C. a. Pb+ (aq) + Mg(s) b. Br() + 2 CI (aq) c. MnO(s) + 4H+ (aq) + Cu(s) Pb(s) + Mg2+ (aq) 2 Br (aq) + Cl(8) 2+ Mn+ (aq) +...
-
One item is omitted in each of the following four lists of income statement data. Determine the amounts of the missing items, identifying them by letter. Chase Company $735,000 Jessup Inc. Osterman...
-
Snoop's Bakery makes and sells cakes and cookies. Snoop Dogg is concerned about the continued losses shown by the cookie segment and wants to see whether or not the line should be dropped. Cookies...
-
When might a manager prefer linear trend technique to simple moving average technique? Explain.
-
You are the auditor of Pacific Foods Limited (PFL), a company that provides food for major corporate events throughout South Australia. The balance date for PFL is 31 st December 2022. On the 15 th...
-
V = Find the transition Matrix 1. [2] ve [3] - [2] 12 [2]
-
Consider the technology you grew up with and how it has changed over time. Think about how technology currently impacts society and student learning. Specifically, address the following prompts: 1....
-
On April 1, Julie Spengel established Spengels Travel Agency. The following transactions were completed during the month. 1. Invested $15,000 cash to start the agency. 2. Paid $600 cash for April...
-
A test car is driven a fixed distance of n miles along a straight highway. (Here n Z+.) The car travels at one mile per hour for the first mile, two miles per hour for the second mile, four miles...
-
With Example 10.9 on page 494 in mind, determine the number of grooves a transmission grating must have if it is to resolve the sodium doublet in the first-order spectrum. Compare the results of both...
-
Sunlight impinges on a transmission grating that is formed with 5000 lines per centimeter. Does the third-order spectrum overlap the second-order spectrum? Take red to be 780 nm and violet to be 390...
-
Suppose that a grating spectrometer while in vacuum on Earth sends 500-nm light off at an angle of 20.0 in the first-order spectrum. By comparison, after landing on the planet Mongo, the same light...
-
If following flowchart was executed, what is the final value of variable x? Start Initialize x-10, k-3 k < 5 COMPUTE x=x+k T COMPUTE x=x+5 End
-
Let sequence (an) be -8, -3, 2, 7, ... (a = -8) a. Find recursive formula for an. b. Find explicit (iterative) formula for an
-
Write a Go program that processes a list of messages using a concurrent function. A main function passes the list of messages to a go function that encrypts each message and send each resulting...

Study smarter with the SolutionInn App


