Calculate the molar solubility of magnesium fluoride (MgF 2 ) in a solution that is 0.250 M
Question:
Calculate the molar solubility of magnesium fluoride (MgF2) in a solution that is 0.250 M in NaF. For magnesium fluoride, Ksp = 5.16 * 10-11.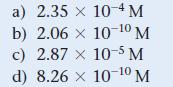
Transcribed Image Text:
a) 2.35 x 10-4 M b) 2.06 x 10-10 M c) 2.87 x 10-5 M d) 8.26 x 10-10 M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
d ...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
51+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Exercises 125, simplify the given expression or perform the indicated operation (and simplify, if possible), whichever is appropriate. 11
-
Calculate the molar solubility of strontium sulfate, SrSO4, in 0.0015 M sodium sulfate, Na2SO4. Solve the equation exactly. See Table 17.1 for Ksp. TABLE 17.1 Solubility Product Constants, Ksp at 25C...
-
a. Calculate the molar solubility of barium fluoride, BaF2, in water at 25C. The solubility product constant for BaF2 at this temperature is 1.0 106. b. What is the molar solubility of barium...
-
Find the point in the first quadrant on the curve y = x + x 1 closest to the origin.
-
Maize Company incurs a cost of $35 per unit, of which $20 is variable; to make a product that normally sells for $58. A foreign wholesaler offers to buy 6,000 units at $30 each. Maize will incur...
-
Lloyd Inc. has sales of $200,000, a net income of $15,000, and the following balance sheet: The new owner thinks that inventories are excessive and can be lowered to the point where the current ratio...
-
The Tuckers owned an RV that they insured through American Family. On August 26, 2012, their RV was struck by lightning and damaged. The Tuckers reported the damage to American Family. In March 2013,...
-
Rachel Rey recently opened her own basketweaving studio. She sells finished baskets in addition to the raw materials needed by customers to weave baskets of their own. Rachel has put together a...
-
1. there are two different schools of thought on how to lower gasoline prices and reduce U.S. dependence on foreign oil. One is to increase supply of oil (drilling offshore), and the other is to...
-
The magnesium and calcium ions present in seawater ([Mg 2+ ] = 0.059 M and [Ca 2+ ] = 0.011 M) can be separated by selective precipitation with KOH. What minimum [OH - ] triggers the precipitation of...
-
Calculate the molar solubility of lead(II) bromide (PbBr 2 ). For lead(II) bromide, K sp = 4.67 * 10 -6 . a) 0.00153 M b) 0.0105 M c) 0.0167 M d) 0.0211 M
-
Summit Systems will pay a dividend of $1.46 this year. If you expect Summits dividend to grow by 5.1% per year, what is its price per share if its equity cost of capital is 11.2%?
-
An illegal contract is valid unless it is executory. (True/False)
-
All individualsregardless of their knowledge, skill, or intelligencemust exercise the same duty of care if they wish to avoid liability for negligence. (True/False)
-
A person who borrows a friends car and fails to return it at the friends request is guilty of conversion. (True/False)
-
The tort of defamation does not occur unless a defamatory statement is made in writing. (True/False)
-
Ed tells customers that he is the best plumber in town. This is fraudulent misrepresentation, unless Ed actually believes that he is the best. (True/False)
-
What are some similarities and differences between skimming pricing, prestige pricing, and above-market pricing?
-
How many years will it take a $700 balance to grow into $900 in an account earning 5%?
-
Design a problem to help other students better understand low-pass filters described by transfer functions. Determine the cutoff frequency of the lowpass filter described by Find the gain in dB and...
-
Find the transfer function V o V s of the circuit in Fig. 14.86 . Show that the circuit is a low-pass filter. 10 H 1F= vo 0.25 2 Vs
-
Using Fig. 14.80 , design a problem to help other students better understand the quality factor, the resonant frequency, and bandwidth of RLC circuits. For the circuits in Fig. 14.80, find the...
-
On May 1, 2025, Crane Company purchased factory equipment for $739700. The asset's useful life in hours is estimated to be 190000. The estimated salvage value is $35000 and the estimated useful life...
-
Superior Company provided the following data for the year ended December 31 (all raw materials are used in production as direct materials): Selling expenses Purchases of raw materials Direct labor...
-
Thermal Rising, Incorporated, makes paragliders for sale through specialty sporting goods stores. The company has a standard paraglider model, but also makes custom-designed paragliders. Management...

Study smarter with the SolutionInn App


