Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction: a. Use the reactions and associated
Question:
Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction:![]()
a. Use the reactions and associated equilibrium constants at body temperature given here to find the equilibrium constant for the reaction just shown.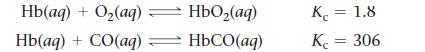
b. Suppose that an air mixture becomes polluted with carbon monoxide at a level of 0.10% (by volume). Assuming that the air contains 20.0% oxygen and that the oxygen and carbon monoxide ratios that dissolve in the blood are identical to the ratios in the air, what is the ratio of HbCO to HbO2 in the bloodstream? Comment on the toxicity of carbon monoxide.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





