Consider a 0.10 M solution of a weak polyprotic acid (H 2 A) with the possible values
Question:
Consider a 0.10 M solution of a weak polyprotic acid (H2A) with the possible values of Ka1 and Ka2 given here.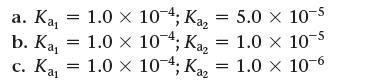
Calculate the contributions to [H3O+] from each ionization step. At what point can the contribution of the second step be neglected?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





