Consider the electrolytic cell: a. Label the anode and the cathode and indicate the halfreactions occurring at
Question:
Consider the electrolytic cell: 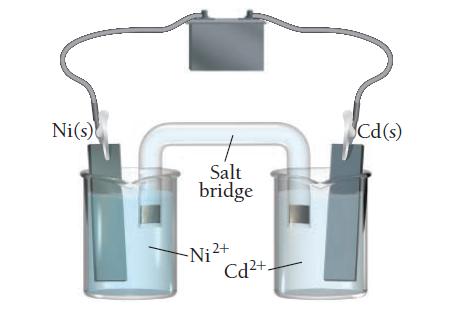
a. Label the anode and the cathode and indicate the halfreactions occurring at each.
b. Indicate the direction of electron flow.
c. Label the terminals on the battery as positive or negative and calculate the minimum voltage necessary to drive the reaction.
Transcribed Image Text:
Ni(s) Salt bridge Cd(s) Hi Ni ²+ Cd²+.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Anode Ni N...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Ray Holt Corporation has retained you as a consultant on accounting policies and procedures. During 2019, the company engaged in a number of treasury stock transactions, having foreseen an...
-
26. Originally from England, Joy received her permanent Canadian resident status three years ago. She lives in Edmonton, where she works as a surgeon major hospital. She travels back to her home...
-
Consider the voltaic cell: a. Determine the direction of electron flow and label the anode and the cathode. b. Write a balanced equation for the overall reaction and calculate E cell . c. Label each...
-
Briefly explain the meaning of the following concepts and terms: greenhouse gases (GHGs), the IPCC, the little ice age, the Keeling graph, telekinetic property of the atmosphere, global weirding,...
-
Johanssen Company uses activity-based costing (ABC). Johansson manufactures outdoor water toys using two activities: plastic injection molding and decal application. Johanssons 2009 total budgeted...
-
Environmental Engineering plc is engaged in the development of an environmentally friendly personal transport vehicle. This will run on an electric motor powered by solar cells, supplemented by...
-
Plaintiff sought to enforce against the defendant estate a promise made by his now-deceased uncle to pay Plaintiff a sum of money if Plaintiff refrained from the use of alcohol and tobacco for a...
-
Tierney Company begins operations on April 1. Information from job cost sheets shows the following. Job 12 was completed in April. Job 10 was completed in May. Jobs 11 and 13 were completed in June....
-
2. Consider the variation of hot-potato routing/coordination routing game discussed in class, where there are three strategies: Payoff Hot Potato Long Path Planned Long-Path | Planned Hot Potato...
-
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Mg b. Cr c. Cu
-
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Zn b. Sn c. Mn
-
Projects A and B are both nearing completion. You are managing a super important project C that requires an immediate input of a resource being used by both projects A and B, but is otherwise...
-
What feature or characteristic of the development process does 'The New Service Development Cycle'diagram emphasize? What is a benefit and a disadvantage of that emphasis? How does the diagram...
-
Define a Bank Reconciliation and why they are done. Why are they important to the company? Discuss which internal control principles you think are the most important to safeguard cash receipts and...
-
References: Valacich, J. S., Schneider, C., & Hashim, M. (2022). Information Systems Today: Managing in the Digital World (9th ed.). Pearson. 9780136735854 2-1. Compare and contrast the...
-
A series of numbers follows the pattern T_(n)=n^(2), where T_(n) represents the value of the term and n represents the position of the term in the series. Find the value of T_(n) when n is 35 .
-
3). Which expression will make a random number generator that can only generate integers between 0 and 1 generate numbers between 94 and 140? A). R*94 - 46 B). R*94+46 C). R*140 46 - D). R*47+95 E)....
-
Answer Question 4 for some other organization, perhaps an organization where you have worked. In Question 4, Figure outlines the operations, finance/accounting, and marketing functions of three...
-
C- Consider the following scenario:- A supermarket needs to develop the following software to encourage regular customers. For this, the customer needs to supply his/her residence address, telephone...
-
Obtain v 1 through v 3 in the circuit of Fig. 2.81. + 1 - ww "2 24 V V3 +) 10 V 12 V +
-
Describe a simple U-tube manometer.
-
Describe a differential U-tube manometer.
-
An instrument, maintained at 118.7 C, has two separate flasks that contain different gases. If the flasks are opened so that the gases are able to mix, what is the final density in flask 2? Flask Gas...
-
Compute the discount on a 10 year simple discount loan if the amount borrowed is $1,190 and the annual simple discount rate is 4.4%.
-
g. Switch to Datasheet View then choose Investment Banking & Asset Management for the Industry field for CompanyID 2 (Des Moines Financial Group).

Study smarter with the SolutionInn App


