Consider the reaction: If a mixture of solid nickel(II) oxide and 0.20 M carbon monoxide comes to
Question:
Consider the reaction: 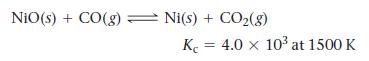
If a mixture of solid nickel(II) oxide and 0.20 M carbon monoxide comes to equilibrium at 1500 K, what is the equilibrium concentration of CO2?
Transcribed Image Text:
NiO(s) + CO(g) Ni(s) + CO₂(g) K 4.0 x 10³ at 1500 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A container has a double wall where the wall cavity is filled with carbon dioxide at room temperature and pressure. When the container is filled with a cryogenic liquid at 100 K the carbon dioxide...
-
A container has a double wall where the wall cavity is filled with carbon dioxide at room temperature and pressure. When the container is filled with a cryogenic liquid at 100 K the carbon dioxide...
-
The formation of tetrafluoroethylene from its elements is highly exothermic: (a) If a mixture of F2, graphite, and C2F4 is at equilibrium in a closed container, will the reaction go to the right or...
-
Find the magnitude and direction of the electric field strength at the point P due to the point charges at A and B as shown in the Figure 1. (k = 9 x 10 Nm/C) +2 C 10 cm A 10 cm Figure 1 B -8 C (5...
-
What is an operational productivity measure? A financial measure?
-
At the beginning of each term, a physics professor named Dr. Zeus shows the class his expectations of them through a demonstration that he calls Lesson #1. He stands at the center of a turntable that...
-
Petitioner Curtis Flowers has been tried six separate times for the murder of four employees of a Mississippi furniture store. Flowers is black; three of the four victims were white. At the first two...
-
PostNews. com offers its subscribers several services, such as an annotated TV guide and local-area information on weather, restaurants, and movie theaters. Its main revenue sources are fees for...
-
3 3. 7 of the coins in a box are nickels. The rest are dimes. If there are 24 dimes, how many nickels are there?
-
For the reaction shown here, K c = 255 at 1000 K. CO(g) + Cl 2 (g) COCl 2 (g) If a reaction mixture initially contains a CO concentration of 0.1500 M and a Cl 2 concentration of 0.175 M at 1000 K,...
-
For the reaction shown here, K c = 0.513 at 500 K. N 2 O 4 ( g) 2 NO 2 (g) If a reaction vessel initially contains an N 2 O 4 concentration of 0.0500 M at 500 K, what are the equilibrium...
-
Tidal Company has significant amounts of trade accounts receivable. In March of this year, Tidal assigned specific trade accounts receivable to Adapted Herb Finance Company on a with-recourse, non...
-
Name the three processes that make up project procurement management.
-
What is the main purpose of monitoring and controlling a project?
-
How might a project leaders understanding of the team members individual motivations affect the projects overall quality?
-
Do small businesses often outsource project work? Why or why not?
-
An important input to the Plan Quality Management process is requirements documentation. This is because: a. the organization will have a uniform set of specific quality requirements that every...
-
Tohono Companys 2013 master budget included the following fixed budget report. It is based on an expected production and sales volume of 20,000 units. Required 1. Classify all items listed in the...
-
Accounting policies and practices that are most important to the portrayal of the companys financial condition and results, and require managements most difficult, subjective, or complex judgments...
-
Two blocks of mass m 1 = 2.5 kg and m 2 = 3.5 kg rest on a double inclined plane with equal angles (Fig. P4.87). The blocks are connected by a string that passes over a pulley, and the blocks are in...
-
A person riding a bicycle on level ground at a speed of 10 m/s throws a baseball forward at a speed of 15 m/s relative to the bicycle at an angle of 35 relative to the horizontal (x) direction. (a)...
-
A car travels along a level road with speed v = 25 m/s (about 50 mi/h). The coefficient of kinetic friction between the tires and the pavement is K = 0.55. (a) If the driver applies the brakes and...
-
Direct labor-hours required to support estimated production Machine-hours required to support estimated production Fixed manufacturing overhead cost Variable manufacturing overhead cost per direct...
-
Manufacturing costs for Davenport Company during 2018 were as follows: Beginning Finished Goods, January 1 Beginning Raw Materials, January 1 Beginning Work in Process, January 1 Direct Labor for the...
-
Work in Process Job 33 Job 34 Balance on 3/1 $ 4,900 3,600 $ 8,500 Source documents revealed the following during March: Materials Requisitions Forms Labor Time Tickets $ 4,400 Job 33 Job 34 $ 2,800...

Study smarter with the SolutionInn App


