Consider the table listing the solubilities of several alcohols in water and in hexane. Which statement best
Question:
Consider the table listing the solubilities of several alcohols in water and in hexane. Which statement best describes the observed trend in terms of intermolecular forces?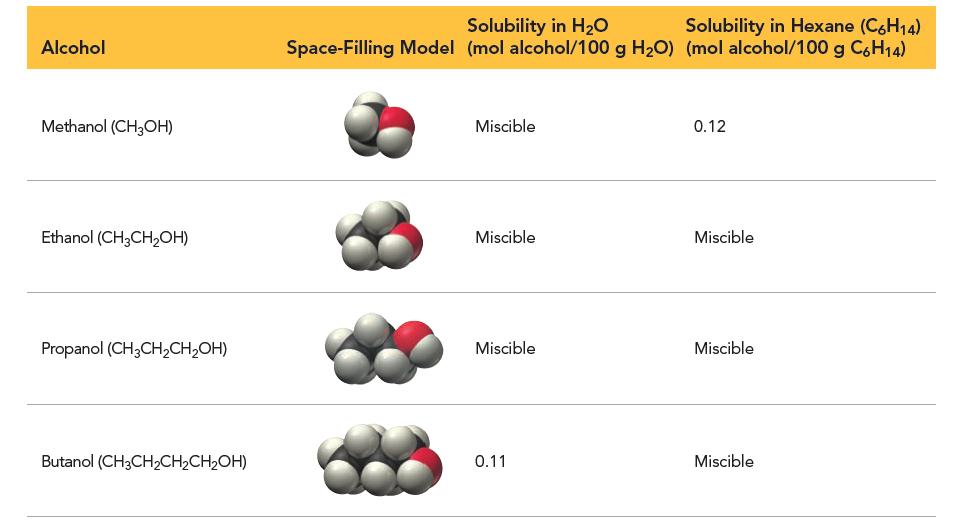
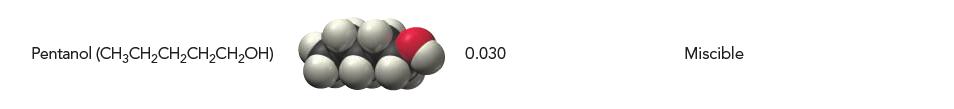
(a) As you move down the list, molecules become more polar, less soluble in water, and more soluble in hexane.
(b) As you move down the list, molecules become more polar, more soluble in water, and less soluble in hexane.
(c) As you move down the list, molecules become less polar, less soluble in water, and more soluble in hexane.
(d) As you move down the list, molecules become less polar, more soluble in water, and less soluble in hexane.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





