Consider this two-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates
Question:
Consider this two-step mechanism for a reaction: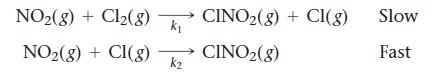
a. What is the overall reaction?
b. Identify the intermediates in the mechanism.
c. What is the predicted rate law?
Transcribed Image Text:
NO₂(g) + Cl₂(8) NO₂(g) + Cl(g) K₁ k₂ CINO₂(g) + CI(g) CINO₂(g) Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a What is the overall reaction The overall reaction is NO2g Cl2g NO2Clg This is because the second s...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCl3) and chlorine: Step 1: Step 2: Step 3: (a) What is the overall reaction? (b) What are the intermediates in...
-
A proposed mechanism for a reaction is C4H9Br C4H9+ + Br2 Slow C4H9+ + H2O C4H9OH2+ Fast C4H9OH2+ + H2O C4H9OH + H3O+ Fast Write the rate law expected for this mechanism. What is the overall...
-
In a hydrocarbon solution, the gold compound (CH3)3AuPH3 decomposes into ethane (C2H6) and a different gold compound, (CH3)AuPH3. The following mechanism has been proposed for the decomposition of...
-
Use lHospitals rule where applicable to find each limit. lim In(ex + 1) 5x
-
Why is actual costing rarely used for product costing?
-
The thermodynamic identify for a one-dimensional system is d = dU fdl When f is the external force exerted on the line and dl is the extension of line. By analog with (32) we form the derivative to...
-
1. Identify an entrepreneur in your area you would like to interview. 2. Contact the person you have selected and make an appointment. Be sure to explain why you want the appointment and to give a...
-
Rolen, Inc., is in the process of preparing the fourth quarter budget for 2010, and the following data have been assembled: The company sells a single product at a price of $25 per unit. The...
-
help me respond to this, Can I request a little insight on this zoom meetings attendees and the intended end result? Will this information conclusion be going back to the County auditors in a report?...
-
Many heterogeneous catalysts are deposited on high-surfacearea supports. Why?
-
Consider this three-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates in the mechanism. c. What is the predicted rate law? k Cl(8) k Cl(g) + CHCl3(8)...
-
First Development Corporation of Kentucky (FDCK) sought to purchase a fifteen-acre parcel of riverfront property owned by Martin Marietta. On May 9, FDCK made an offer to purchase the property for...
-
What is the difference between the required and expected rates of return of a common stock?
-
Why would a convertible bond increase much more in value than a bond that is not convertible?
-
Why is the search for new profitable projects so important?
-
Maria wants to sponsor her son for high school. She plans to withdraw $2,000 at the beginning of each year for 10 years, from a special account that will pay 12 percent annually. In order to achieve...
-
Differentiate between realized and expected returns.
-
Recall the chapters opening feature involving Omar Soliman and Nick Friedman, and their company, College Hunks Hauling Junk LLC. Assume that Omar and Nick, partners in the company, decide to expand...
-
Swifty company is a publicly held corporation whose $1 par value stock is actively traded at $30 per share. The company issued 3400 shares of stock to acquire land recently advertised at $93000. When...
-
An engine that operates at the maximum efficiency possible takes in 6.0 MJ of heat from a hot reservoir at 327C and exhausts waste heat to a cold reservoir at 127C. How much work does it perform?
-
An engine is proposed that is to operate between 250C and 60C with an efficiency of 40 percent. Will the engine perform as predicted? If not, what would its maximum efficiency be?
-
Is it correct to say that a refrigerator produces cold? If not, why not?
-
What is partial dependency? With what normal form is it associated?
-
Suppose you had an entity with the following attributes (see posted data dictionary): INV_NUMBER,LINE_NUMBER, INV_DATE, CUS_CODE, P_CODE, P_PRICE, P_DESCRIPTION, LINE_UNITS, LINE_PRICE,V_CODE, V_NAME...
-
Find the volume of the solid that lies under the surface z = x+ y and above the rectangle R [1,4] x [0, 1] in the xy-plane.

Study smarter with the SolutionInn App


