Examine the phase diagram for iodine shown in Figure 12.39(a). FIGURE 12.39a What state transitions occur as
Question:
Examine the phase diagram for iodine shown in Figure 12.39(a).
FIGURE 12.39a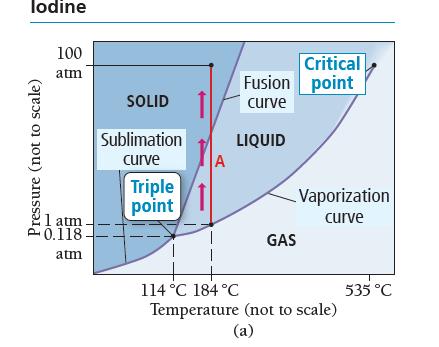
What state transitions occur as we uniformly increase the pressure on a gaseous sample of iodine from 0.010 atm at 185 °C to 100 atm at 185 °C? Make a graph, analogous to the heating curve for water shown in Figure 12.36. Plot pressure versus time during the pressure increase.
Figure 12.36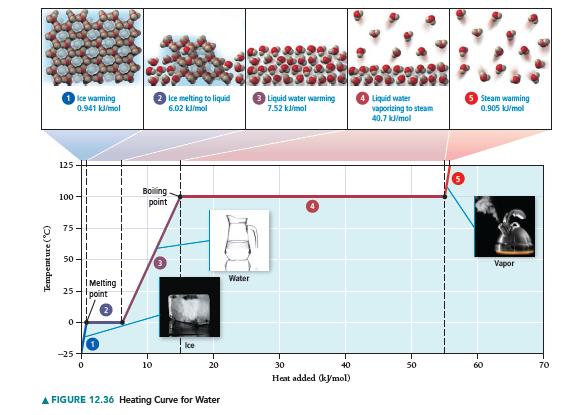
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





