Find H rxn for the reaction: Use these reactions with known Hs: 3 C(s) + 4 H(g)
Question:
Find ΔHrxn for the reaction: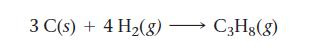
Use these reactions with known ΔH’s: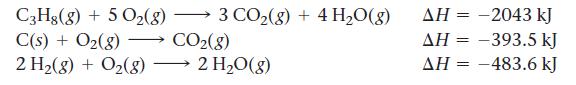
Transcribed Image Text:
3 C(s) + 4 H₂(g) → C3H8(8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To work this and other Hesss law problems manipulate the reactions with known AHs in such a way as t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Can someone please summarize the case study below: if you know about the case IKEA Looks to Further Penetrate the U.S. Market 10 CASE Synopsis: IKEA is known around the world for its stylish,...
-
Allergic reactions to poison ivy can be miserable. Plant oils cause the reaction. Researchers at the Allergy Institute did a study to determine the effects of washing the oil off within 5 minutes of...
-
Coulometric titration of sulfite in wine. Sulfur dioxide is added to many foods as a preservative. In aqueous solution, the following species are in equilibrium: Bisulfite reacts with aldehydes in...
-
What is the "ALDI Way" and what was its quest? Take out costs; eliminate complexity Survival; make enough money to pay overhead costs Increase market share; find more prospective customers Build...
-
Fantastic Props, Inc., designs and fabricates movie props such as mock-ups of star-fighters and cybernetic robots. The companys balance sheet as of January 1, the beginning of the current year,...
-
ABC, health care Uppervale Health Center runs three programs: (1) alcoholic rehabilitation (2) drug addict rehabilitation, and (3) aftercare (counseling and support of patients after release from a...
-
Follow the steps below to prove the LLN without using CLT. (a) Let \(X\) be a random variable with mean \(\mu\) and variance \(\sigma^{2}\). Then for any real number \(\alpha>0,...
-
BizCon, a consulting firm, has just completed its first year of operations. The company's sales growth was explosive. To encourage clients to hire its services BizCon offered 180-day...
-
What ways can I make my Relational Data Model conversion meet the following requirements listed below? Am I on the right track? What corrections do I need to address? ERD: My Conversion of ERD onto a...
-
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one of the calorimeters, q rxn = -12.5 kJ and in the other q rxn =...
-
Consider the reactions: What is H for the reaction 2 B 3 C? A 2 B A 3C ,
-
Rent control today looks far different from the rent freeze New York City enacted after World War II. Most rent controls today simply restrict annual rent increases and guarantee landlords a "fair...
-
What is a dual-purpose test?
-
Name the four different types of substantive procedures discussed.
-
What is the biggest risk with payables? Why?
-
What are three possible tests of controls over the payroll cycle?
-
Are there any audits where the auditor would perform no substantive audit procedures? Explain your answer.
-
Assume that Eagle Corporation has issued 10%, participating cumulative preferred stock with a total par value of $22,000 and common stock with a total par value of $44,000. Therefore, the preferred...
-
Multiple Choice Questions: 1. The largest component of aggregate demand is? a. Government purchases. b. Net exports. c. Consumption. d. Investment. 2. A reduction in personal income taxes, other...
-
The strain at point A on the bracket has components ε x = 300 (10 -6 ), ε y = 550 (10 -6 ), γ xy = -650 (10 -6 ), ε z = 0. Determine (a) the...
-
The strain at point A on a beam has components ε x = 450(10 -6 ), ε y = 825(10 -6 ), γ xy = 275(10 -6 ), ε z = 0. Determine (a) the principal...
-
The strain at point A on the pressure-vessel wall has components ε x = 480(10 -6 ), ε y = 720(10 -6 ), γ xy = 650(10 -6 ). Determine (a) the principal strains at...
-
Consider your chosen careers, relationships, and interactions with others. Regardless of your chosen major or career field, it is important to be a competent communicator. Through your knowledge of...
-
5 Due to erratic sales of its sole product-a high-capacity battery for laptop computers-PEM, Incorporated, has been experiencing financial difficulty for some time. The company's contribution format...
-
The Shirt Shop had the following transactions for T-shirts for Year 1, its first year of operations. Jan. 20 Purchased 410 units $12= $4,920 Apr. 21 Purchased 130 units $13= 1,690 July 25 Purchased...

Study smarter with the SolutionInn App


