Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(CN) 2 ]-
Question:
Find the equilibrium constant at 298 K for the reaction.![]()
The Kf for [Cu(CN)2]- = 1.0 * 1024 and the rest of the data needed are in Appendix II.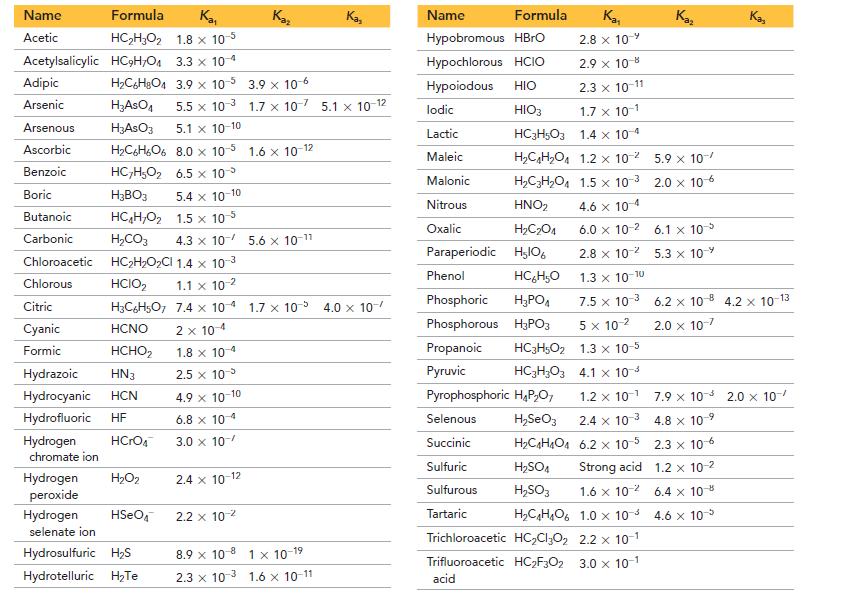
Transcribed Image Text:
[Ag(CN)₂] (aq) + Cu(s) [Cu(CN)₂] (aq) + Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
5...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the following exercises, use the vertical line test to determine which graphs show relations that are function x m II
-
Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(NH 3 ) 2 ]+ = 6.3 * 10 10 and the rest of the data needed are in Appendix II. 2 [Cu(NH3)2](aq) = [Cu(NH3)4]+ (aq) + Cu(s)
-
Muharraq Co. paid the following different costs in 2020: 1. $2,000,000 to acquire a machine to be used in the R&D projects. The machine has a 4- year useful life (Muharraq Co. started using the...
-
A health care facility in a metropolitan area is interested in the efficiency of its laboratory turnaround time. Based on data collected over last year, the mean turn around time was found to be 55...
-
The long-term debt section of Midwest Corporation's balance sheet at the end of its fiscal year, December 31, 2010, is as follows: Using the effective interest method, prepare entries in journal form...
-
The accounts receivable clerk for Vandalay Industries prepared the following partially completed aging-of-receivables schedule as of the end of business on November 30. The following accounts were...
-
99 percent per month. In 2014, Boling resolved his suit against the gas can manufacturer. Shortly thereafter, Prospect sent Boling a Schedule of Purchases, asserting that Boling owed Prospect...
-
The following are Sullivan Corp.'s comparative balance sheet accounts at December 31, 2012 and 2011, with a column showing the increase (decrease) from 2011 to 2012. Additional information:1. On...
-
Suppose A is a 2 by 2 matrix such that the image of the unit circle under the linear transformation given by A is: 2.0 1.5 1.0 2.0 1.5 1.0 0.5 0.5 0.0 0.0 -0.5 -0.5 -1.0 -1.0 -1.5 -1.5 -2.0 -2.0 -2.0...
-
Hydrogen can be in both the octahedral and tetrahedral holes for lanthanum. Determine the percentage of the holes that are filled if the formula is LaH 2.76 .
-
When a part is made by pressing together powdered metal, no metal is wasted. In contrast, metal is typically scrapped after a metal part is cut from a solid metal plate. If a circular part with a...
-
THS produces two products from different combinations of the same resource. Details of the products are shown below: THS is preparing the production plan for next month. The maximum resource...
-
You are considering quitting your job where you earn $3,400 per month and opening a new business. The cost of renting an office is $2,200 per month, hiring employees would cost $3,300 per month, and...
-
Post Lab Task Assemble and execute a program to add five unsigned hexadecimal numbers stored in consecutive memory locations starting from the address labelled Numbers using DCD directive as shown...
-
Develop the idea of a project/product/ segment . Present the feasibility study of your idea. . Present details of the budgeting plan for the first five years of your operation. . Show the logical...
-
fashion is the subculture topic 1 what are the characteristic of the subgroup itself? why does subgroup exist? what its purpose?2 what if any are the cost of entry to the subculture 3 why do...
-
Given monthly mortgage payments of $1100, heating costs of $250/month, property taxes of $150/month, and a car loan of $600/month, determine the Total Debt Service Ratio (TDS) if the client has a...
-
Benson and Orton are partners who share income in the ratio of 1:3 and have capital balances of $70,000 and $30,000 respectively. Ramsey is admitted to the partnership and is given a 40% interest by...
-
What is the back work ratio? What are typical back work ratio values for gas-turbine engines?
-
Two point charges are located as shown in Figure P18.7, with charge q 1 = +2.5 C at x = -3.0 m, y = 0, and charge q 2 = +4.0 C at x = +1.0 m, y = +2.0 m. An electron is now taken from a point very...
-
The electric field in a particular region of space is found to be uniform, with a magnitude of 400 N/C and parallel to the +y direction. (a) What is the change in electric potential energy of a...
-
Three point charges Q 1 = 2.5 ?C, Q 2 = 4.5 ?C, and Q 3 = -3.5 ?C are arranged as shown in Figure P18.9. What is the total electric potential energy of this system? Figure P18.9 ? y Q Q2 L Q1 I L =...
-
2. For how many integers n with 0 n 100 is the quantity integer? n(n - 1)(2n-1) equal to an 12
-
(3) Find a matrix A = R2 and an invertible diagonal matrix D = R such that A has the null space property of order 1 but AD does not.
-
10 1 1 2. (10 points) For the filter h(s, t) given by h(s, t) = 20 , -2 the upper left pixel 1 0 is assumed to have indices (-1, -1). a.) Explicitly write the output image g(m,n) as a sum of scaled...

Study smarter with the SolutionInn App


