For each reaction, calculate H rxn , S rxn , and G rxn at 25 C and
Question:
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C?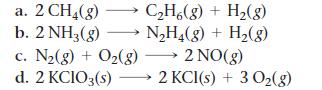
Transcribed Image Text:
a. 2 CH₂(g) b. 2 NH3(g) C₂H6(g) + H₂(g) N₂H₂(g) + H₂(g) c. N₂(g) + O₂(g) →→→ 2 NO(g) d. 2 KCIO3(s)→→→→→2 KCl(s) + 3 O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To calculate Hrxn Srxn and Grxn we need the standard enthalpy of formation Hf values and standard en...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that your company has decided to invest RM316 million in a 5-year construction project. The project is expected to be sold for RM200 million once completed. Estimate the Internal Rate of...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 62. How well do the values of G calculated this way compare to those calculated from H and S? Which of the...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 61. How do the values of G calculated this way compare to those calculated from H and S? Which of the two...
-
Why is it important to have a defined project scope? Why is it important to make sure there is agreement about the scope? Is there anything in the "Why Should You Use the WBS?
-
Discuss the distinguishing features of the Japanese distribution system.
-
A company rewards its production department employees for meeting budgeted cost levels by giving out bonuses. If the departments costs exceed the budget, employees do not get a bonus. What problems...
-
Use the Hubble expansion relation (9.1.1), the temperature scaling relation (9.1.3), and the energy density relation before the electron-positron annihilation (9.3.6b) to show that the temperature as...
-
Edge Companys production vice president believes keeping up-to-date with technological changes is what makes the company successful and feels that a machine introduced recently would fill an...
-
Antelopes, native to Africa and Asia, range in size from 30 cm to over 180 cm at the shoulder, with most between 90-120 cm. This is related to the humerus length. The data below gives the length and...
-
Determine G for the reaction: Use the following reactions with known G rxn values: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g)
-
In photosynthesis, plants form glucose (C 6 H 12 O 6 ) and oxygen from carbon dioxide and water. Write a balanced equation for photosynthesis and calculate H rxn , S rxn , and G rxn at 25 C. Is...
-
Let f(x) = 6x 2 - 2 and g(x) = x 2 - 2x + to find the following values. g(r + h)
-
In a well-organized, well-supported paragraph of at least two hundred words, respond to one of Maia Szalavitz's main arguments. Do you agree or disagree? Refer briefly but specifically to the reading...
-
What are your personal experiences with targeted advertising via the Internet? Do you find these forms of advertising effective, useful, useless, invasive or something else entirely? What do you...
-
Not all managers are leaders and not all leaders are managers'. Critically evaluate this statement in light of what you have learnt about management and leadership in this module to date. In your...
-
briefly explain scene in titanic movie clip" you going to cut her meat too call" with social, cultural and analyze the body language and language alignment between meaning and non verbal...
-
How do politics and black humor coincide with one another? How are the films Dr. Strangelove or: How I Stopped Worrying and Love the Bomb and/or Bulworth examples of this sub-genre of comedy?
-
Aligning Human Resources Systems with Business Strategy: It seems to be very common sense to not have HR practices that are competing with one another. Why does this happen and what would be your...
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
Consider the flagpole in Figure P8.29. If the flagpole has a mass of 20 kg and length 10 m and the angle the cable makes with the pole is ?? = 25, what are the magnitude and direction of the force...
-
A painter is standing on the ladder (mass 40 kg and length 2.5 m) in Figure P8.30. There is friction between the bottom of the ladder and the floor with S = 0.30, but there is no friction between...
-
Consider again the ladder in Problem 30. What is the sign of the torque on the ladder due to the force from the wall? Data from Problem 30 A painter is standing on the ladder (mass 40 kg and length...
-
Garret Company has provided the following selected information for the year ended December 31, 2022: Cash collected from customers was $784,000. Cash received from stockholders in exchange for common...
-
Which of the six steps for creating a strategic marketing plan refers to all the energy and work an employee puts in to get a strategy into action?
-
Explain the difference between GET and POST HTTP methods.

Study smarter with the SolutionInn App


