For each reaction, calculate H rxn , S rxn , and G rxn at 25 C and
Question:
For each reaction, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C and state whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from 25 °C?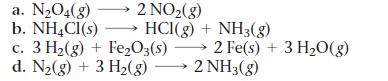
Transcribed Image Text:
2 NO₂(g) HCI(g) + NH3(g) a. N₂04(g) b. NH4Cl(s) c. 3 H₂(g) + Fe₂O3(s) d. N₂(g) + 3 H₂(g) →→→ 2 NH3(g) 2 Fe(s) + 3 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a AHIxn 572 kJ ASn 1758 JK AGxn48 x 10 Jmol nonspontaneous becomes spo...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that your company has decided to invest RM316 million in a 5-year construction project. The project is expected to be sold for RM200 million once completed. Estimate the Internal Rate of...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 62. How well do the values of G calculated this way compare to those calculated from H and S? Which of the...
-
Use standard free energies of formation to calculate G at 25 C for each reaction in Problem 61. How do the values of G calculated this way compare to those calculated from H and S? Which of the two...
-
The labor force participation rate is increasing in Japan because... a) there are fewer unemployed people b) the working age population is increasing c) retirement ages are increasing d) more women...
-
NAFTA has been in existence for several yearshow has it done? Review Exhibit 9.6, which discusses the initial provisions of the agreement, and, using the Internet, evaluate how well the provisions...
-
Diaz Company prepared a budget last period that called for sales of 14,000 units at a price of $12 each. Variable costs per unit were budgeted to be $5. Fixed costs were budgeted to be $21,000 for...
-
Its Intoxicating Inc., is a Pennsylvania corporation that manufactures and distributes cosmetic products to various retailers. Maritim is a German company that owns and operates hotels throughout...
-
Serial Bond Debt Service Fund Journal Entries and Financial Statements. As of December 31, 2010, New Town had $9,500,000 in 4.5 percent serial bonds outstanding. Cash of $509,000 is the debt service...
-
Simplify the given expression and write the answer with only positive exponents. -113 P 3/7 7 3 54x y z 2 w) 3) (w + 2) (3w + w 4 2 (-3x +7x+8)= (x * + 7x *-12x-1) - 5 27-7-28 20-x-22 712-24-8...
-
In photosynthesis, plants form glucose (C 6 H 12 O 6 ) and oxygen from carbon dioxide and water. Write a balanced equation for photosynthesis and calculate H rxn , S rxn , and G rxn at 25 C. Is...
-
Methanol (CH 3 OH) burns in oxygen to form carbon dioxide and water. Write a balanced equation for the combustion of liquid methanol and calculate H rxn , S rxn , and G rxn at 25 C. Is the...
-
What problems result if each stage of a supply chain views its demand as the orders placed by the downstream stage? How should firms within a supply chain communicate to facilitate coordination?
-
Why should we consider merging Strategic planning and human resources planning a strength for an HR professional? any examples to support the answer?
-
The town of Cross Plains lies in a peculiar location where one side of the town (the West Side) is immune from floods while the other side (yes, the East Side) is hit randomly by heavy flooding when...
-
How can corporate leaders shape the culture of their organization and who can change this culture (explain).
-
Identify a specific concept with implications related to human resources--such as immigration, diversity, ethics, sexual harassment, recruitment and selection, compensation, or privacy--and compare...
-
Netflix Operations and Logistics, Human Resource Management (HRM), and Information Technology (IT). As a global business leader, it is imperative to understand all concepts and internal working in an...
-
Do you favour anti-gouging laws as a means of protecting consumers from high prices following natural disasters, such as Hurricane Katrina in New Orleans? If so, why? If not, why not?
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
Consider again the cube in Problem 34 (Fig. P8.34), but now assume the force is applied along a horizontal line that is 0.20 m above the floor. At what value of F will the crate begin to tip? Figure...
-
Repeat Problem 34, but assume the force F is applied at the corner of the cube and at an angle of 30 above the horizontal direction. At what value of F will the crate begin to tip? Data from Problem...
-
Wheelbarrows are designed so that a person can move a massive object more easily than if he simply picked it up. If the person using the wheelbarrow in Figure P8.37 is able to apply a total vertical...
-
When an organization integrate entrepreneurship and marketing should customer focus be a key driving force for innovation and entrepreneurship within an organization?
-
A bank offers to syndicate a Eurodollar loan for an MNC. The MNC has a credit risk rating of AA. Loan Type: Bullet Principal: USD 21 million Maturity: 4 years Upfront Syndication Fee: 1.95% Interest...
-
How do you leverage advanced machine learning algorithms, such as deep learning or ensemble methods, within the data mining process to extract complex patterns and insights from large and...

Study smarter with the SolutionInn App


