Gold can be plated out of a solution containing Au 3+ according to the half-reaction: What mass
Question:
Gold can be plated out of a solution containing Au3+ according to the half-reaction:![]()
What mass of gold (in grams) is plated by a 25-minute flow of 5.5 A current?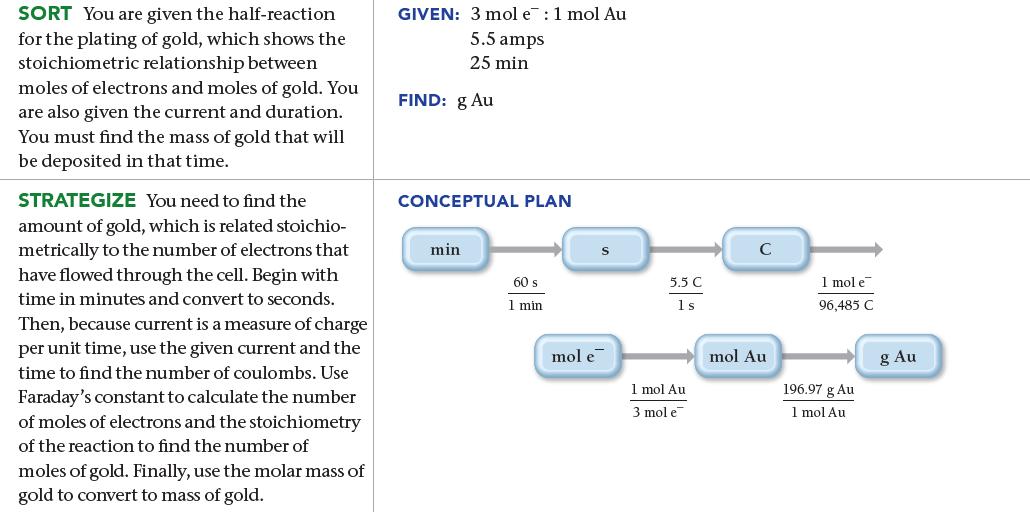
Transcribed Image Text:
3+ Au³+ (aq) + 3 e Au(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
25 min 60 X 1 min ...View the full answer

Answered By

Milan Mondal
I am milan mondal have done my Msc in physics (special astrophysics and relativity) from the University of burdwan and Bed in physical science from the same University.
From 2018 I am working as pgt physics teacher in kendriya vidyalaya no2 kharagpur ,west bengal. And also I am doing advanced physics expert in chegg.com .also I teach Bsc physics .
I love to teach physics and acience.
If you give me a chance I will give my best to you.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Aluminum can be plated out of a solution containing A l3+ according to the half-reaction: A l3+ (aq) +3e - Al (s) What mass of aluminum (in grams) will be plated by the flow of 50 A of current 10...
-
Silver nitrate solutions are often used to plate silver onto other metals. What is the maximum amount of silver (in grams) that can be plated out of 4.8 L of an AgNO 3 solution containing 3.4% Ag by...
-
A 50.0-mL portion of a solution containing 0.200 g of BaCl2 2H2O is mixed with 50.0 mL of a solution containing 0.300 g of NaIO3. Assume that the solubility of Ba(IO3)2 in water is negligibly small...
-
Write a Fortran program that creates an integer array with values -123, -4, 5, 67, 890, and 12345. Prints out the array in several formatted ways. a. Print each element, on its own line, using a...
-
Chemical Pro uses an automated mixing machine in its Mixing Department to combine three raw materials into a product called Triogo. On average, each unit of Triogo contains $3 of Material X, $6 of...
-
Amy Rockwell was a brilliant but penniless electrical engineer. She had designed a new type of cogeneration plant that she believed had great commercial potential. On January 15, she approached...
-
Identify the research scenario, including the general area of focus. Develop a hypothetical research scenario that would necessitate the use of the Mixed Method A-B-A Design. The research will be...
-
Doug Casey is in charge of planning and coordinating next springs sales management training program for his company. Doug listed the following activity information for this project: a. Draw a project...
-
Crystal Corporation earned net income of $ 9 0 0 , 0 0 0 in 2 0 2 3 . It has a complex capital structure as follows: 1 2 , 0 0 0 shares outstanding of 8 % , $ 1 0 0 preferred stock, and 1 7 0 , 0...
-
What is the definition of the standard cell potential (E cell )? What does a large positive standard cell potential imply about the spontaneity of the redox reaction occurring in the cell? What does...
-
Find E cell for an electrochemical cell based on the following reaction with [MnO 4 ] = 2.0 M, [H + ] = 1.0 M, and [Ag + ] = 0.010 M. Ecell for the reaction is +0.88 V. MnO4 (aq) + 4H+ (aq) + 3 Ag(s)...
-
How does prudence relate to neutrality?
-
Carol has a 2018 Kia Sorento that has a current value of $27,000. The car was purchased on the date of March 1, 2020, at an initial purchase price of $32,000. They paid $3,000 upfront in cash for it...
-
Mr. Seyed Ramsey has employment income of $105000. He owns shares that are listed on the Toronto Stock Exchange. These shares have a fair market value of $186000 and an adjusted cost base of 42000....
-
2. Describe how you: a) Identified and negotiated the group's needs, objectives, and activities. b) Encouraged group members to voice their ideas, needs and expectations whilst respecting the values...
-
The probability distribution of returns for stocks RARE and SURE are given below. Probability TRARE 0,5 -%2 0,1 %10 0,4 %15 ISURE %20 %12 %2 a) Please find the expected return and standard deviation...
-
as a member of the UOW community, I am responsible for maintaining the quality and rigour of the institution. I am found in violation of this agreement that I will be subject to university Academic...
-
Identify and analyze three basic patterns in indigeneous religions. Use examples from African religions to support your answer.
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
Utilizing Eq. (11.38), show that F -1 {F{(x)}} = Æ(x). ik(x x') k 6( ') (11.38) 2
-
Given F{(x)}, show that F{(x - x 0 )} differs from it only by a linear phase factor.
-
Prove that * h = h * directly. Now do it using the convolution theorem.
-
What sophisticated considerations are required to go beyond the simple partitioning of data into tables and delve into the intricacies of normalization?
-
Is this statement true? Justify. Tautology is a mistake committed by a researcher who has empirical evidence about an association between two or more variables at the level of individual behaviour...
-
MTV Corporation has 7 percent coupon bonds on the market with a par of $1,000 and 8 years left to maturity. The bonds make semi-annual interest payments. If the market interest rate on these bonds is...

Study smarter with the SolutionInn App


