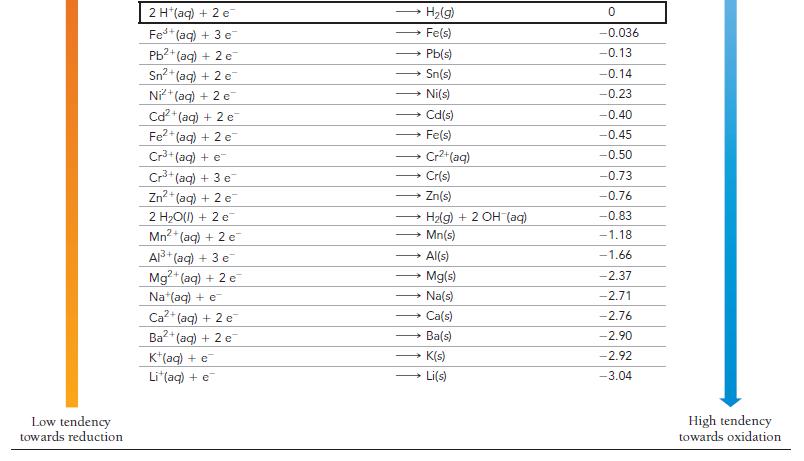
How can Table 20.1 be used to predict whether or not a metal will dissolve in HCl?
Question:
How can Table 20.1 be used to predict whether or not a metal will dissolve in HCl? In HNO3?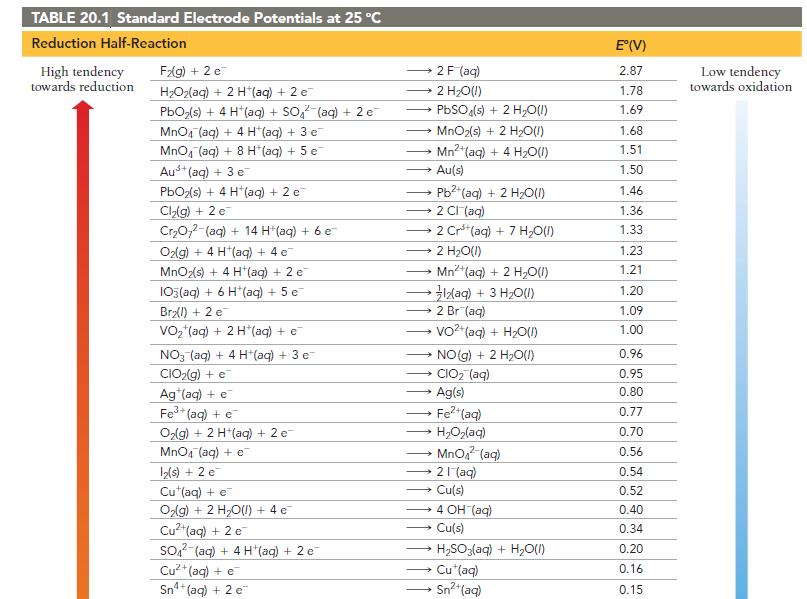
Transcribed Image Text:
TABLE 20.1 Standard Electrode Potentials at 25 °C Reduction Half-Reaction High tendency towards reduction F₂(g) + 2 e H₂O₂(aq) + 2 H+(aq) + 2 e PbO₂ (s) + 4 H*(aq) + SO² (aq) + 2 e MnO4 (aq) + 4 H (aq) + 3 e MnO4 (aq) + 8 H(aq) + 5 e Aus+ (aq) + 3 e PbO₂(s) + 4 H*(aq) + 2 e Cl₂(g) + 2 e Cr₂O72-(aq) + 14 H+ (aq) + 6 e- O₂(g) + 4 H (aq) + 4 e MnO₂(s) + 4 H*(aq) + 2 e 103(aq) + 6 H*(aq) + 5 e Br₂(l) + 2 e VO₂ (aq) + 2 H*(aq) + €¯ NO3(aq) + 4 H+(aq) + 3 e CIO₂(g) + e Ag (aq) + e Fe³+ (aq) + e O₂(g) + 2 H+(aq) + 2 e- MnO4 (aq) + e 12(s) + 2 e™ Cu (aq) + e O₂(g) + 2 H₂O(l) + 4e¯ Cu²+ (aq) + 2 e SO2 (aq) + 4 H(aq) + 2 e Cu²+ (aq) + e Sn²4+ (aq) + 2 c → Au(s) 2 F (aq) 2 H₂O(l) PbSO4(s) + 2 H₂O(l) MnO2(s) + 2 H₂O(l) Mn²+ (aq) + 4H₂O(1) → 2 CI (aq) - Pb2+ (aq) + 2 H₂O(l) - 2 Cr³+ (aq) + 7 H₂O(l) 2 H₂O(1) Mn²+ (aq) + 2 H₂O(l) • }zaq) + 3 HO(I) 2 Br (aq) VO²+ (aq) + H₂O(l) → CIO₂ (aq) Ag(s) Fe²+ (aq) HyOzlag) MnO₂ (aq) 21 (aq) → NO(g) + 2 H₂O(1) → Cu(s) → 4 OH (aq) Cu(s) H₂SO3(aq) + H₂O(l) Cu*(aq) Sn²+ (aq) E°(V) 2.87 1.78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.96 0.95 0.80 0.77 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0.15 Low tendency towards oxidation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Table 201 titled Standard Electrode Potentials at 25C can be used to predict whether a metal will di...View the full answer

Answered By

Amos Kiprotich
I am a wild researcher and I guarantee you a well written paper that is plagiarism free. I am a good time manager and hence you are assured that your paper will always be delivered a head of time. My services are cheap and the prices include a series of revisions, free referencing and formatting.
4.90+
15+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A version of simple exponential smoothing can be used to predict the outcome of sporting events. To illustrate, consider pro football. Assume for simplicity that all games are played on a neutral...
-
Every year, millions of high school students apply and vie for acceptance to a college of their choice. For many students and their parents, this requires years of preparation, especially for those...
-
An online retailer is offering a new line of running shoes. The retailer plans to send out an e-mail with a discount offer to some of its existing customers and wants to know if it can use data...
-
PROJECT SUMMARY: You have been asked to submit a proposal to a client, Sara Johnson, who is moving the small firm to a new office location. The proposal is on the analysis and design of the office...
-
Reuse Cookware, Inc., manufactures sets of heavy-duty pots. It has just completed production for August. At the beginning of August, its Work in process Inventory account showed direct materials...
-
NTG Telecommunications, which was interested in purchasing a computer network for its office, received a promotional letter from IBM regarding IBMs PC Server 310, Small Business Solution, a computer...
-
Use the technique discussed in Section9.4 to develop a GEE approach for zeroinflated Poisson model for count responses in longitudinal studies. Section9.4: 9.4 Marginal Models for Longitudinal Data...
-
Newton Inc. uses a calendar year for financial reporting. The company is authorized to issue 9,000,000 shares of $10 par common stock . At no time has Newton issued any potentially dilutive...
-
The figure shows a schematic diagram of a simple mass spectrometer, consisting of a velocity selector and a particle detector and being used to separate singly ionized atoms (q+e 1.602e-19 C) of gold...
-
Which metal can be used as a sacrificial electrode to prevent the rusting of an iron pipe? a) Au b) Ag c) Cu d) Mn
-
Does a large positive electrode potential indicate a strong oxidizing agent or a strong reducing agent? What about a large negative electrode potential?
-
Write the complete ionic equation and the net ionic equation for the reaction that occurs between aqueous solutions of: (a) Silver nitrate and potassium iodide (b) Lithium sulfate and silver acetate
-
Shares of stock of Sweet & Sour Co., a domestic corporation, held as investment by Benjo bought at P1,000,0000 cost in mid 2022, are listed and traded in the local stock exchange. A year later, Benjo...
-
Accounting A man claims he had no idea the car he bought had entered the island illegally. If this is the case, what provisions have been established to allow the man to keep or receive value for...
-
What are the principal objectives of virtual memory management in modern operating systems, and how do techniques like demand paging and page replacement algorithms such as LRU (Least Recently Used)...
-
Write a program in C++ to write the following pattern in a text file "pattern.txt" and read the same pattern from the file and display it. |-|-|-| -|-|-|-|- |-|-|-|-|-| -1-1-1-1-1-1- -|-|-|-|-|-|-|-...
-
General Bonds Corp. (GBC) has a pension liability of $100M. The duration of this liability is 15 years. On the asset side of its balance-sheet, GBC has a 100% bond portfolio that consists of two...
-
Consider the following demand schedule for bottles of water: PRICE (P) Quantity Demanded by Consumers (bottles / month) $0.50 ................1,100 1.00...............1,050 1.50...............1,000...
-
Determine the optimal use of Applichem's plant capacity using the Solver in Excel.
-
Blood flows through an artery of diameter d and length L, and the pressure difference between the ends of the artery is P. If the diameter is reduced by a factor of two and the pressure difference is...
-
What will happen if water is poured on top of the mercury and ball bearing of Problem 78 such that the ball bearing is completely submerged in the water? Will the ball bearing (a) sink farther into...
-
A small, square plank of oak floats in a beaker half full of water. The piece of oak is 6.0 cm on a side and 3.0 cm thick and floats on its side as shown in Figure P10.80. (a) Find the location of...
-
what ways does normalization contribute to the broader objectives of database governance, data quality management, and regulatory compliance, by promoting standardized data structures, minimizing...
-
Quad Enterprises is considering a new three-year expansion project that requires an initial fixed asset investment of $2.32 million. The fixed asset will be depreciated straight-line to zero over its...
-
While testing the voltage of a component used in a microcomputer for the Hertz Company, the data listed were obtained. 1. Construct x-bar chart and R chart of these data. Determine whether the...

Study smarter with the SolutionInn App


