Hydrogen sulfide (H 2 S) smells like rotten eggs; its smell can be detected at concentrations as
Question:
Hydrogen sulfide (H2S) smells like rotten eggs; its smell can be detected at concentrations as low as 0.02 ppm. Well water, which is drawn from underground depths of 30–250 meters (100–800 feet), is sometimes contaminated with hydrogen sulfide.
The contamination arises from bacterial decomposition of sulfur-containing organic and inorganic matter present in the oxygen-deficient underground environment. Some H2S gas dissolves in the water that is pumped for drinking supplies, where it is detected by taste at concentrations as low as 0.05 ppm.
Municipalities remove H2S to improve water taste and to prevent the accumulation of toxic H2S gas within the system.
The first table that follows lists hydrogen sulfide solubility in water when the partial pressure of hydrogen sulfide is 1.00 atm.
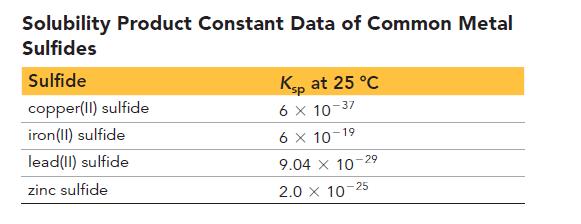
The second table lists solubility product constant data for common metal sulfides.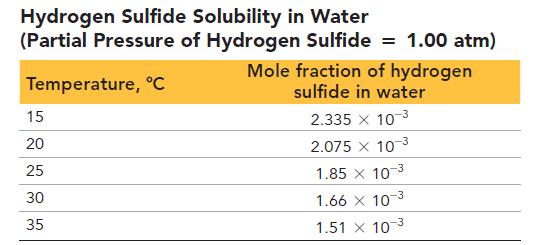
Use the information provided in the tables to answer the following questions:
a. What can you conclude about the temperature dependence of H2S solubility in water?
b. Suppose that water analysis indicates that your well’s water contains 1.8 mg H2S per liter of well water. Using Henry’s law (Chapter 14) and the data in the first table, calculate the partial pressure of H2S, in atm, that exists above your well water at 25 °C.
c. Air containing H2S at 20 ppm (by volume) and below is considered safe to breathe. Is the air above the solution in part b safe to breathe?
d. A water technician recommends that you treat your well water with hydrogen peroxide, H2O2, in order to convert H2S to sulfate (which does not have an offensive odor or taste), according to the following unbalanced reaction:![]()
What volume of a 3.0% by mass solution of hydrogen peroxide is required to treat 955 liters of your well water? (Assume that no other species are present to react with the H2O2 and that the density of the hydrogen peroxide solution is 1.0 g/mL.)
e. Hydrogen sulfide is a weak diprotic acid that ionizes in water to form hydrogen sulfide (HS¯) and sulfide (S2¯). Sulfide dissolved in water can be precipitated as an insoluble metal sulfide, according to the following reaction:![]()
Which metal in the second table most effectively removes hydrogen sulfide from water?
f. What volume, in mL, of a 0.500 M solution of the metal ion you chose in part e is required to remove the hydrogen sulfide present in 955 liters of your well water (from part b)?
g. What mass, in grams, of the metal sulfide (from part f) precipitates?
Step by Step Answer:






