LP gas burns according to the exothermic reaction: What mass of LP gas is necessary to heat
Question:
LP gas burns according to the exothermic reaction: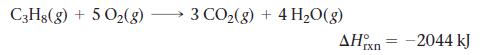
What mass of LP gas is necessary to heat 1.5 L of water from room temperature (25.0 °C) to boiling (100.0 °C)? Assume that during heating, 15% of the heat emitted by the LP gas combustion goes to heat the water. The rest is lost as heat to the surroundings.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





