Nitromethane (CH 3 NO 2 ) burns in air to produce significant amounts of heat. How much
Question:
Nitromethane (CH3NO2) burns in air to produce significant amounts of heat.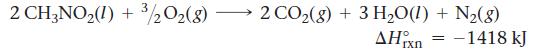
How much heat is produced by the complete reaction of 5.56 kg of nitromethane?
Transcribed Image Text:
2 CH3NO2(I) + 3/2O2(g) 2 CO2(g) + 3 H2O(1) + N2(g) rxn = –1418 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
64...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Magnesium burns in air to produce magnesium oxide, MgO, and magnesium nitride, Mg 3 N 2 . Magnesium nitride reacts with water to give ammonia. Mg 3 N 2 (s) + 6H 2 O(l) 3Mg(OH) 2 (s) + 2NH 3 (g) What...
-
In addition to each process having unique fixed costs, each process also has unique variable costs. In other words, the processing cost per unit is different for each process. Process variation is a...
-
Disk A, with a mass of 2.0 kg and a radius of 50. cm, rotates clockwise about a frictionless vertical axle at 30. rev/s. Disk B, 1.0 kg with a radius of 100. cm, rotates counterclockwise at 20. rev/s...
-
Make a comparison of the following insurance coverage premium bids and determine from a costing standpoint, which offer is better. Show your calculations: Assume that you, as a risk manager would...
-
The internal control procedures in Payton Company make the following provisions. Identify the principles of internal control that are being followed in each case. (a) Employees who have physical...
-
Rod AB is attached to a collar at A and is fitted with a small wheel at B which rolls on a circular surface. Knowing that when = 60? the velocity of the collar is 1.2 ft/s downward, determine, at...
-
Epic Systems is a Wisconsin health care software company. In 2014, Epic introduced a company policy that required employees to use individual arbitration in any disputes. Jacob Lewis, an Epic...
-
The following transactions, adjusting entries, and closing entries were completed by Robinson Furniture Co. during a three-year period. All are related to the use of delivery equipment. The...
-
(1 point) A local polka band wants to make and sell CDs of its songs. Suppose it costs $ 1000 to record, S 450 to edit, and S 350 for album artwork, and suppose each CD that is manufactured will cost...
-
Titanium reacts with iodine to form titanium(III) iodide, emitting heat. Determine the masses of titanium and iodine that react if 1.55 * 10 3 kJ of heat is emitted by the reaction. 2 Ti(s) + 3 1(g)...
-
What mass of natural gas (CH4) must burn to emit 267 kJ of heat? CH4(g) + 2 Oz(g) COz(g) + 2H,O(g) AH = -802.3 kJ xxn
-
Express each as a sum, difference, or multiple of logarithms. Wherever possible, evaluate logarithms of the result. log 10 (1000x 4 )
-
First, let's see what we can learn about retirement needs from the TVM calculations we know how to do. You expect to need $75,000 a year in retirement and you expect your retirement to last 35 years....
-
42. A retailer's net profit (NP) on the sale of merchandise is determined by subtracting overhead (O) expenses from the merchandise markup (M): NPM O. Determine M when - NP $122 and O = $74. =
-
Determine the price of a $1.4 million bond issue under each of the following independent assumptions: 1. Maturity 10 years, interest paid annually, stated rate 6%, effective (market) rate 8%. 2....
-
Management accounting literature is abundant with discussions and commentaries on the changing roles of management accountants. The advent of Johnson and Kaplan's, 'Relevance Lost,' has certainly...
-
Suppose you buy a $ 7 0 0 , 0 0 0 house with ' no money down' with a 3 0 year, fixed - rate 7 % mortgage paid month starting TODAY! After 3 0 years you've finally paid off your mortgage....YEAH How...
-
Kaley Perry invests $8,559.48 now for a series of $1,000 annual returns, beginning one year from now. Kaley will earn a return of 8% on the initial investment. How many annual payments of $1,000 will...
-
A company has the following incomplete production budget data for the first quarter: In the previous December, ending inventory was 200 units, which was the minimum required, at 10% of projected...
-
One classic problem in quantum mechanics is the harmonic oscillator. In this problem a particle is subjected to a one-dimensional potential (taken to be along x) of the form V (x) x 2 , where x ....
-
A crude model for the molecular distribution of atmospheric gases above the Earths surface (denoted by height, h) can be obtained by considering the potential energy due to gravity: In this...
-
Another use of distribution functions is determining the most-probable value, which is done by realizing that at the distribution maximum the derivative of the distribution function with respect to...
-
Graph the following function. g(x) = (x - 10)
-
3. a. b. Solve the system by inverting the coefficient matrix and using Theorem 1.6.2 of the textbook. -x-2y-32 = 0 w+x+4y+4z = 7 w+3x+7y+9z = 4 -w-2x-4y - 6z = 6 Determine conditions on the b's, if...
-
1. If 5,000 earns Php86.00 interest in 15 months, how much is the rate of interest? 2. If the interest rate is 7.5% per year and the principal is Php2,500, determine the amount of interest for 20...

Study smarter with the SolutionInn App


