Refer to Figure 12.36 to answer each question. a. A sample of steam begins on the line
Question:
Refer to Figure 12.36 to answer each question.
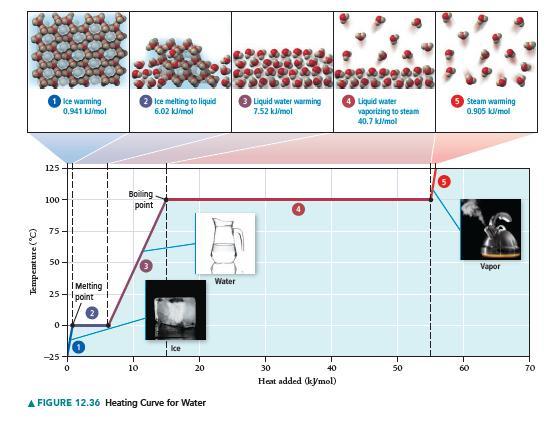
a. A sample of steam begins on the line segment labeled 5 on the graph. Is heat absorbed or released in moving from the line segment labeled 5 to the line segment labeled 3? What is the sign of q for this change?
b. In moving from left to right along the line segment labeled 2 on the graph, heat is absorbed, but the temperature remains constant. Where does the heat go?
c. How would the graph change if it were for another substance (other than water)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





