Silver iodide crystallizes in the zinc blende structure. The separation between nearest-neighbor cations and anions is approximately
Question:
Silver iodide crystallizes in the zinc blende structure. The separation between nearest-neighbor cations and anions is approximately 325 pm, and the melting point is 558 °C. Cesium chloride, by contrast, crystallizes in the structure shown in Figure 13.14.
Even though the separation between nearest-neighbor cations and anions is greater (348 pm), the melting point of cesium chloride is higher (645 °C). Explain.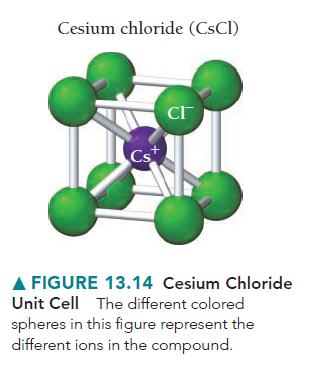
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





