Sketch the titration curve from Problem 123 by calculating the pH at the beginning of the titration,
Question:
Sketch the titration curve from Problem 123 by calculating the pH at the beginning of the titration, at one-half of the equivalence point, at the equivalence point, and at 5.0 mL beyond the equivalence point. Pick a suitable indicator for this titration from Table 18.1.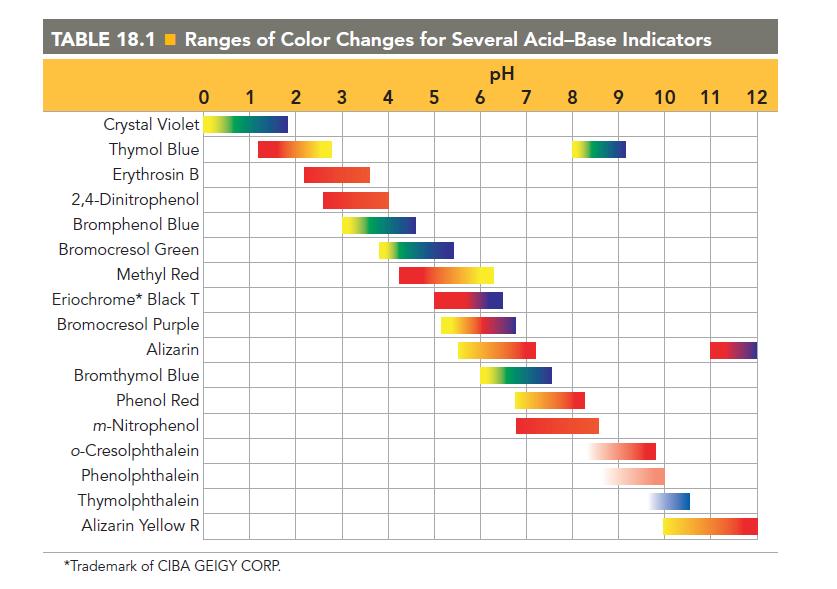
Problem 123
A 0.552-g sample of ascorbic acid (vitamin C) was dissolved in water to a total volume of 20.0 mL and titrated with 0.1103 M KOH. The equivalence point occurred at 28.42 mL. The pH of the solution at 10.0 mL of added base was 3.72. From this data, determine the molar mass and Ka for vitamin C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





