The emission of NO 2 by fossil fuel combustion can be prevented by injecting gaseous urea into
Question:
The emission of NO2 by fossil fuel combustion can be prevented by injecting gaseous urea into the combustion mixture. The urea reduces NO (which oxidizes in air to form NO2) according to the reaction: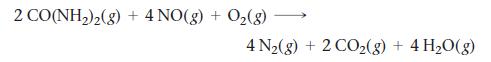
Suppose that the exhaust stream of an automobile has a flow rate of 2.55 L/s at 655 K and contains a partial pressure of NO of 12.4 torr. What total mass of urea is necessary to react completely with the NO formed during 8.0 hours of driving?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





