The enthalpy of solution for cesium fluoride is -36.8 kJ/mol. What can you conclude about the relative
Question:
The enthalpy of solution for cesium fluoride is -36.8 kJ/mol. What can you conclude about the relative magnitudes of ΔHsolute and ΔHhydration?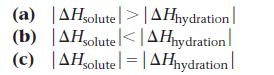
Transcribed Image Text:
(a) AHsolute>|AHhydration (b) AHsolute<| AHhydration (c) |AHsolute = |AHhydration |
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
b You ...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The enthalpy of solution for NaOH is -44.46 kJ/mol. What can you conclude about the relative magnitudes of the absolute values of Hsolute and Hhydration , where Hsolute is the heat associated with...
-
What can you conclude about the relative risk of investing in the United States versus Japan from Figure 7.4?
-
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of lithium iodide endothermic or exothermic? b. What can you conclude about the relative magnitudes...
-
1. Resolve Class C 192.168.23.36 /27 2. Design DMZ minimumof 4 servers assigning an IP addressfor all devices 3. List and explain 4 primary servers that will be in the DMZ 4. Resolve Class C 192.168...
-
What is value-chain analysis? What role does it play in strategic cost analysis?
-
Would it be possible in principle for the earth to escape from the solar system?
-
Continuing to focus on evidence associated with the act, concealment, and conversion, use the evidentiary material to continue the examination. In addition, as the examiner also start to think of...
-
Physical Phitness, Inc. operates three divisions, Weak, Average, and Strong. As it turns out, the Weak division has the lowest operating income, and the president wants to close it. "Survival of the...
-
What are some similarities and differences between the Microsoft SQL Server, Oracle Database, Amazon Aurora, IBM Db2, and MySQL relational database vendors?
-
A solution is prepared by dissolving 17.2 g of ethylene glycol (C 2 H 6 O 2 ) in 0.500 kg of water. The final volume of the solution is 515 mL. Calculate the concentration of the solution in each...
-
Why do two ideal gases thoroughly mix when combined? What drives the mixing?
-
A Blu-ray disc spins at 1530 r/min when the innermost edge is being read by the laser, and gradually slows to a rate of 630 r/min at the outer edge. Use an inequality to express the angular velocity ...
-
In 2019, Windsor Knott, an employee of the Victoria Tie Company, was seriously injured in the factory stockroom. He was hospitalized for 30 days and lost partial use of his left hand. During his...
-
Ron and Gayle, both over 65 years of age, have the following sources of income: Ron and Gayle have itemized deductions of $16,000. Compute their taxable income. Consulting income interest income...
-
Robert Careless was injured while working on the production line on July 8, 2019. He received the following payments as a result of his serious injury: During 2019, Robert earned $16,000 in wages....
-
Rodney and Alice Jones have three small children, ranging in age from 5 to 10. One child is blind and needs special care. Rodney works as an accountant for a large CPA firm and has gross income of...
-
Show that B d (t) as defined in Problem 21.1 is almost surely a continuous process. Problem 21.1 Suppose that the tenor structure T 1 , T 0 ,..., T N is equispaced, witht = T n T n 1 and T 1 = 0....
-
Georgia Orchards produced a good crop of peaches this year. After preparing the following income statement, the company believes it should have given its No. 3 peaches to charity and saved its...
-
Find the volume of the described solid S. A frustum of a right circular cone with height h, lower base radius R, and top radius r -r- --R
-
In the fabricated enlargement shown in Fig. 6.29, the pressure at A is 25.6 psig and the pressure at B is 28.2 psig. Calculate the volume flow rate of oil (sg = 0.90). Direction of flow 5-in inside...
-
For the special fabricated reducer shown in Fig. 6.28, the pressure at A is 50.0 psig and the pressure at B is 42.0 psig. Calculate the velocity of flow of water at point B. Flow 1-in inside diameter...
-
For the siphon shown in Fig. 6.27, calculate (a) the volume flow rate of oil from the tank and (b) the pressures at points A, B, C, and D. +B 3,0 m Oil (sg - 0.86) 10.0 m 50-mm OD x 15-mm wall 25-mm...
-
An adult's life can be divided into time spent working (W) and time spent in retirement (R). Although it is plainly difficult to know how long you will spend in each period of life, this question...
-
Find the magnitude of vector AB (AB), if the vector has an initial point at A(-3, -5) and a terminal point at B(4,-4)?
-
2. Use linear approximation to approximate 10 - 1.0012/(5.002).

Study smarter with the SolutionInn App


