The first six elements of the first transition series have the following number of stable isotopes: Explain
Question:
The first six elements of the first transition series have the following number of stable isotopes: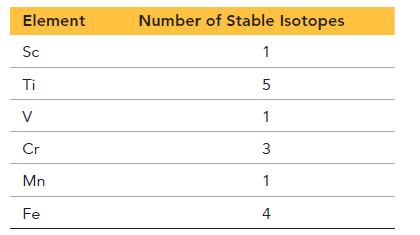
Explain why Sc, V, and Mn each have only one stable isotope while the other elements have several.
Transcribed Image Text:
Element Sc Ti V Cr Mn Fe Number of Stable Isotopes 1 5 1 3 1 4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Sc V and Mn each have odd numbe...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Which of the following questions involve microeconomics, and which involve macroeconomics? In each case, explain your answer. a. Why did consumers switch to smaller cars in 2008? b. Why did overall...
-
Explain how costs are assigned to activities.
-
1. Prepare a memo to Golopolus, summarizing the new safety guidelines that affect the Rockingham product line and requesting his authorization for implementation. 2. Mind your own business. Golopolus...
-
In question 1, identify the marketing as opposed to the business strategy. Data From Question 1: What is a business strategy? Do you agree with the definition proposed? Illustrate your answer with...
-
The management of Red Robin Co. is reevaluating the appropriateness of using its present inventory cost flow method, which is average-cost. They request your help in determining the results of...
-
Q. In order to attain economic progress, humans use the natural resources of the planet. Nature is fashioned into products for human consumption. This economic activity is needed to create jobs and...
-
Suppose that an 85.0-gram laboratory animal ingests 10.0 mg of a substance that contained 2.55% by mass Pu-239, an alpha emitter with a half-life of 24,110 years. a. What is the animals initial...
-
Calculate the quantity of energy produced per mole of U-235 (atomic mass = 235.043922 amu) for the neutron-induced fission of U-235 to produce Te-137 (atomic mass = 136.9253 amu) and Zr-97 (atomic...
-
Using integration, determine the area and the centroidal distance y of the shaded area. Then, using the second theorem of Pappus-Guldinus, determine the volume of a paraboloid formed by revolving the...
-
Chuck is standing statically on his right foot and you want to determine the force on his achilles tendon. Chuck has a body mass of 80 kg. The horizontal distance from his ankle to his achilles...
-
In 1981, Israel bombed a nuclear reactor in Iraq to prevent Saddam Hussein from developing a nuclear bomb that could strike Israel. Was Israel acting in lawful self-defense when it attacked a nuclear...
-
Newdex has net income of $3,100,000 (INCLUDING the effect of expected out-of-pocket costs) and 1,000,000 shares outstanding. It needs to raise $5,100,000 in funds for a new asset. Its investment...
-
A shipper of fresh fish and seafood, Mr. Den Denmark, delivered a refrigerated van of goods to the G.V. Suntide Vessel at the port of St. George's, Grenada on July 15 th , 2022 for shipment to...
-
Firm X needs to net $7,600,000 from the sale of common stock. Its investment banker has informed the firm that the retail price will be $23 per share, and that firm X will receive $20.00 per share....
-
What criteria can be developed for assessing the audit process? What are the critical steps in the audit process that can be evaluated?
-
How is use of the word consistent helpful in fraud reports?
-
Why is gravity considered a fundamental force whereas the force a bat exerts on a ball is not?
-
Of the following, the shortest is a. 1 mm b. 0.01 in. c. 0.001 m d. 0.001 ft
-
What is the difference between spring and neap tides? Under what circumstances does each occur?
-
For this activity, explore the cost associate and the economic order quantity formula that determines a company's inventory reorder point. Find an article in an MRO-related journal in the Hunt...
-
Perform the indicated operation and simplify if possible. Assume any factors you cancel are not zero. 5 v2-7v-18 v+2 (v+2)(v-9)
-
35. Use the synthetic division and the Remainder Theorem to evaluate P(c) P(x)=-3x5 + 4x4 - 3x3 - 10x + 4x-7 C = -1 30

Study smarter with the SolutionInn App


