The graph shows the distribution of molecular velocities for two different molecules (A and B) at the
Question:
The graph shows the distribution of molecular velocities for two different molecules (A and B) at the same temperature. Which molecule has the higher molar mass? Which molecule has the higher rate of effusion?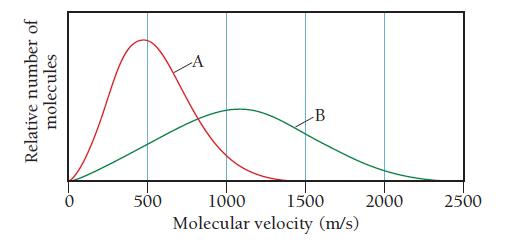
Transcribed Image Text:
Relative number of molecules 500 -A -B 1000 1500 Molecular velocity (m/s) 2000 2500
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
A has the hi...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The graph shows the distribution of molecular velocities for the same molecule at two different temperatures (T 1 and T 2 ). Which temperature is greater? Explain. Relative number of molecules 0 T T...
-
The following chart plots the net present value (NPV) of projects A and B at different discount rates. The projects have similar risk and are mutually exclusive. a. What is the significance of the...
-
Armani Weavings Limited (AWL) obtained a long term loan of Rs. 300 million from Dunhill Bank Limited (DBL) against the charge over its factory buildings. The charge was duly registered with the...
-
On April 1, 2014, Briggs Corp. purchases a 24-month property insurance policy for $72,000. The policy is effective immediately. Assume that Briggs prepares adjusting entries only once a year, on...
-
Speedy Parcel Service operates a fleet of delivery trucks in a large metropolitan area. A careful study by the companys cost analyst has determined that if a truck is driven 120,000 miles during a...
-
a. 1. How many shares of common stock had been issued as of August 1, 2008? 2. How many shares of common stock were outstanding as of August 1, 2008? 3. What share number is used to compute earnings...
-
The account balances for the year ended December 31,2010, for Williams Industries are listed next: Requirements 1. Prepare Williams Industries multi-step income statement. 2. Prepare Williams...
-
On January 1, 2011, Quick Stop, a convenience store, purchased a new soft-drink cooler. Quick Stop paid $25,780 cash for the cooler. Quick Stop also paid $1,090 to have the cooler shipped to its...
-
Sales Cost of goods sold Accounts receivable 2021 $ 553,188 273,512 26,830 2020 $ 359,213 177,614 20,978 2019 $ 287,370 144,011 19,599 2018 $ 197,505 97,827 11,534 2017 $ 148,500 72,765 10,143...
-
Which postulate of the kinetic molecular theory breaks down under conditions of high pressure? Explain.
-
A sample of N 2 O effuses from a container in 42 seconds. How long will it take the same amount of gaseous I 2 to effuse from the same container under identical conditions?
-
Which of the following individuals is most likely to be audited? a. Connie has a $20,000 net loss from her unincorporated business (a cattle ranch). She also received a $200,000 salary as an...
-
Data from the last nine decades for the broad U.S. equity market yield the following statistics: average excess return, 8.3%; standard deviation, 20.1%. what must have been the average coefficient of...
-
Worldwide Satellites Inc. (WSI), a U.S. multinational, is evaluating a new project in France. This project will cost EUR 25 million in fixed assets (satellite machine) and will require an increase in...
-
Ki-Hung purchased one call on XYZ stock at an exercise price of $25. The market price of XYZ stock when Ki-Hung purchased the call was $24 a share. XYZ is currently priced at $30 a share. Ki-Hung...
-
Identify and discuss two or three ways that a Christian view of change is similar to that of a cognitive behavioral approach. Support your position with references to the videos or reading. Based on...
-
9. Suppose that Expectation theory holds. Assume that face values of all bonds here is $1,000. In the market, 1. Price of 1 year zero coupon is 970 today. 2. Price of 2 year zero coupon is 930 today....
-
An economic contraction (recession) is now well under way and the Fed plans to use all facilities at its command to halt the decline. Describe the measures that it may take.
-
F.(3e* -2x 3 sin(2x)) is equal to 2 3 Cos 8. IT 3, t (4+@ 2 3, 1+o 1 4 Cos 4 4 1 3. 1 +4cos V7 (1+o 4 1 4 Cos 4 1+0 4-
-
Determine the maximum moment at C caused by the moving loads. 6 k 4 k 2k 3 ft 4 ft 3 ft -20 ft - -30 ft-
-
Determine the absolute maximum live shear and absolute maximum moment in the jib beam AB due to the 10-kN loading. The end constraints require 0.1 m ¤ x ¤ 3.9 m. 4 m 'A 10 kN
-
The truck has a mass of 4 Mg and mass center at G 1 , and the trailer has a mass of 1 Mg and mass center at G 2 , Determine the absolute maximum live moment in the bridge in Problem 669 if the...
-
Health Technology of the Future PLEASE USE THIS RESOURCES BELOW FOR YOUR RESPONSES. Mesk (2019). 20 Medical Technology Advances: Medicine in the Future....
-
Ivanhoe Ltd. purchased machinery on January 1, 2023, for $75,200. The machinery is estimated to have a residual value of $4,800 after a useful life of eight years. (a) Calculate the 2023 depreciation...
-
Marin Company makes several products, including canoes. The company reports a loss from its canoe segment (see below). All its variable costs are avoidable, and $317,500 of its fixed costs are...

Study smarter with the SolutionInn App


