The mechanism shown is proposed for the gas-phase reaction, 2 N 2 O 5 4 NO
Question:
The mechanism shown is proposed for the gas-phase reaction, 2 N2O5 → 4 NO2 + O2. What rate law does the mechanism predict?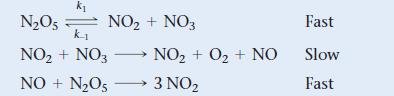
a) Rate = k[N2O5]
b) Rate = k[N2O5]2
c) Rate = k[N2O5]0
d) Rate = k[NO2][NO3]
Transcribed Image Text:
k₁ N₂O5 k₁ NO₂ + NO3 NO + N₂O5 NO₂ + NO3 NO₂ + O₂ + NO 3 NO₂ Fast Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a R...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 -- 4 NO2 + O2. The rate law is first order in N2O5. At the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the...
-
The reaction 2NO(g) + O2(g) 2NO2(g) exhibits the rate law Rate = k[NO]2[O2] Which of the following mechanisms is consistent with this rate law? a. NO + O2 NO2 + O Slow O + NO NO2 Fast b. NO + O2 ...
-
The mechanism shown is acted upon by the force P. Derive an expression for the magnitude of the force Q required for equilibrium. OE
-
For the given year, find the standard quotas for the New York City boroughs given in Table 17.5 in Problems 23-28. Assume there are eight council seats. Table 17. 5 1990 Year Total 1790 49 1800 81...
-
At May 31, 2014, the accounts of Mantle Company show the following. 1. May 1 inventoriesfinished goods $12,600, work in process $14,700, and raw materials $8,200. 2. May 31 inventoriesfinished goods...
-
Launch Failure. A 7500-kg rocket blasts off vertically from the launch pad with a constant upward acceleration of 2.25 m/s2 and feels no appreciable air resistance. When it has reached a height of...
-
Under what conciitions might an independent accountant report on the application of accounting principles?
-
The board of directors of Cable Plus authorizes the issue of $7,000,000 of 6%, 20-year bonds payable. The semiannual interest dates are May 31 and November 30. The bonds are issued on May 31, 2014,...
-
(Compound annuity) You plan on buying some property in Florida 11 years from today. To do this, you estimate that you will need $45,000 at that time for the purchase. You would like to accumulate...
-
What is the rate law for the elementary step Cl + CO ClCO ? (a) Rate = k[Cl] (b) Rate = k[CO] (c) Rate = k[ClCO] (d) Rate = k[Cl][CO]
-
Write integrated rate laws for zero-order, first-order, and secondorder reactions of the form A products.
-
Determine the optimal order quantity and total annual inventory cost for cups in Problem 34 if the carrying cost is 5% of the price of a box of cups.
-
Gallatin Carpet Cleaning is a small, family - owned business operating out of Bozeman, Montana. For its services, the company has always charged a flat fee per hundred square feet of carpet cleaned....
-
On January 1 , 2 0 2 4 , Wright Transport sold four school buses to the Elmira School District. In exchange for the buses, Wright received a note requiring payment of $ 5 2 8 , 0 0 0 by Elmira on...
-
Gibson Corporation produces products that it sells for $ 1 8 each. Variable costs per unit are $ 3 , and annual fixed costs are $ 3 2 8 , 5 0 0 . Gibson desires to earn a profit of $ 4 5 , 0 0 0 ....
-
The call option for a European stock index has a strike price of RM 2 , 1 6 0 and a time to expiration of 0 . 2 5 years.Determine the lower bound for the option price given a risk - free rate of 5...
-
In 2 0 1 7 , the population in the US was estimated at 3 2 5 . 1 million. The population in 2 0 1 8 was estimated to be 3 2 6 . 8 million. Assuming that the population growth is compounding annually...
-
Florida Orange manufactures orange juice. Last months total manufacturing costs for the Saratoga operation included: Direct materials ....... $ 400,000 Direct labor .......... 35,000 Manufacturing...
-
Bonus shares can be issued out of revenue reserves. True/False?
-
Beginning with v g = dÏ/dk prove that dv Vg = -X?
-
Show that First prove that v g = dv/d(1/λ). c d(1/n) d(1/A) Og
-
With the previous problem in mind show that And then since prove that Check this expression by confirming that the units are correct. dn v|1 An d(1/A) || d dv d (1/) d(1/) d
-
You invest $7000 at Capitol Banks who offers an 10 year CD at an annual rate of 5% using simple interest. What is the total value of your investment after 10 years?
-
Carla Vista Inc. manufactures a single product in a continuous processing environment. All materials are added at the beginning of the process, and conversion costs are incurred uniformly throughout...
-
Discuss any challenges your company has encountered in international trade over the past five years. Explain.

Study smarter with the SolutionInn App


