These images represent the first-order reaction A B initially and at some later time. The rate
Question:
These images represent the first-order reaction A → B initially and at some later time. The rate law for the reaction is Rate = 0.010 s-1 [A]. How much time has passed between the two images?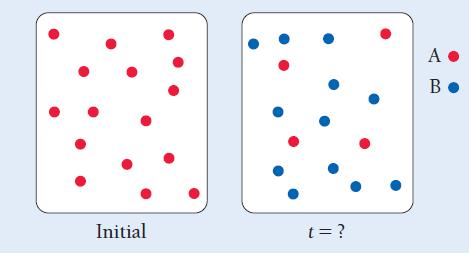
a) 69 s
b) 139 s
c) 60 s
d) 12.5 s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





