This reaction has an equilibrium constant of K p = 2.26 * 10 4 at 298 K.
Question:
This reaction has an equilibrium constant of Kp = 2.26 * 104 at 298 K.![]()
Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium.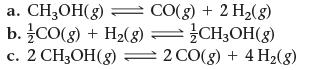
Transcribed Image Text:
CO(g) + 2 H₂(g) = CH3OH(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 442 x 105 react...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. 2 COF2(g) = CO(g) +...
-
A particular reaction has an equilibrium constant of K p = 0.50. A reaction mixture is prepared in which all the reactants and products are in their standard states. In which direction does the...
-
The following reaction has an equilibrium constant Kc equal to 3.07 104 at 24oC. For each of the following compositions, decide whether the reaction mixture is at equilibrium. If it is not, decide...
-
The RRR Company has a target current ratio of 2.4. Presently, the current ratio is 3.3 based on current assets of $6,567,000. If RRR expands its inventory using short- term liabilities (maturities...
-
Explain the important factors to consider for capital investment decisions relating to advanced technology and P2 opportunities.
-
The financial statements for Matts Hats, Inc., appear shown below. Required: 1. Prepare common income statements to be used for horizontal analysis for Matts Hats for 2007 to 2009. 2. Indicate why...
-
Use the Hawkeye Gymnastics data in Exercise 16-22 to compute a. New borrowing or payment of long-term notes payable, with Hawkeye having only one long-term note payable transaction during the year b....
-
The income statement of Kneale Transport Inc. for the year ended December 31, 2014, reported the following condensed information: Kneale's statement of financial position contained the following...
-
Sometimes we choose to buy a product from a firm pursuing a cost leadership strategy, sometimes from one pursuing a differentiation strategy. Is this accurate and why? Discuss experience with the...
-
A chemist trying to synthesize a particular compound attempts two different synthesis reactions. The equilibrium constants for the two reactions are 23.3 and 2.2 * 10 4 at room temperature. However,...
-
Ethene (C 2 H 4 ) can be halogenated by this reaction: where X 2 can be Cl 2 (green), Br 2 (brown), or I 2 (purple). Examine the three figures representing equilibrium concentrations in this reaction...
-
Which two components make up the total return to an investor in a share of stock?
-
You borrow 300,000 to buy a new house. The loan is a conventional 30-year loans and payment is made at the end of each month (so will you make more than just 30 payments, right!) What should be the...
-
A driver is initially 340 miles from home, traveling toward home on a straight interstate at 55 miles per hour. (a) Write a formula for a function that models the distance between the driver and home...
-
If you are financing a new car with a loan that requires 15% interest compounded monthly over a 4 year term. If you make payments $150 at the end of each month and your down payment will be $1,500,...
-
A load is being lifted by moving pulley A downward. If Pulley A starts at the same elevation as pulley B from rest and begins moving downward with a speed of VA = 2t m/s, determine the speed of the...
-
1. In 2023, Rex Ltd., a public company using IFRS, completed a research project initiated in 2022, and a successful patent is obtained. The research phase to complete the project are $75,000. The...
-
Use the annual report of Carnival Corporation for the 2007 scal year to answer the following questions. This information can be found on either the annual report or the SEC 10-K ling at...
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
Evaluate R eq looking into each set of terminals for each of the circuits shown in Fig. 2.103 . a. b. 62 ww- 3 k2 2 k2 6 k2 ww 6 k2
-
Find i and V o in the circuit of Fig. 2.100 . 80 2 24 Q ww 25 2 30 : 20 20 V V. 20 2 60
-
Calculate V o and I o in the circuit of Fig. 2.99. 30 70 200 V 20 5
-
Ekiya, who is single, has been offered a position as a city landscape consultant. The position pays $140,600 in wages. Assume Ekiya has no dependents. Ekiya deducts the standard deduction instead of...
-
ABC123 Inc has decided to purchase 100% the voting shares of DEF456 for $400,000 in cash on July 1, 2019. On the date, the balance sheets of each of these companies were as follows: ABC123 Inc...
-
Harbor Island Investments (HII) is a discount brokerage firm offering clients investment advice, trading services, and a variety of mutual funds for investment. Hill has collected the following...

Study smarter with the SolutionInn App


