Use formal charge to choose the best Lewis structure for CH 3 SOCH 3 . H :O:
Question:
Use formal charge to choose the best Lewis structure for CH3SOCH3.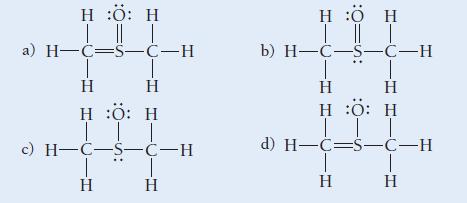
Transcribed Image Text:
H :O: H | || | a) H=C=5-C-H T Η Η H :O: H III © H-C-5-C-H Η Η H :O H | || | b) H-C-5-C-H Η Η H :O: H ||| d) H-C=s-C-H Η T Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
H O...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The algorithm below determines q, r EN such that y = q2 +r and r < 2, where y, z N. DIVIDE(y, z) 1 ry 2 q 0 3 4 5 6 7 8 9 10 11 12 while wy do w- 2w while w > z do q2q [w/2] W- if w
-
Briefly describe some common information system controls that need to be implemented by business managers, not IS professionals.
-
You have been assigned to a project to determine if a new investment should be made. your company uses a capital structure of 30% debt and 70% equity. the debt currently pays 8.5% interest; the...
-
Table illustrates the quantities, marginal costs, average variable costs, and average costs of a competitive firm. Refer to table 4. How much is the unit profit at price =$30? [the problem is based...
-
Describe some generic types of record keys in typical accounting databases. Are such keys simple or complicated?
-
A thin ring is loaded by two equal and opposite forces F in part a of the figure. A free-body diagram of one quadrant is shown in part b. This is a statically indeterminate problem, because the...
-
What is a NDI?
-
This problem continues the Daniels Consulting situation from Problem P4-40 of Chapter 4. Daniels Consulting performs systems consulting. The company has also begun selling accounting software and...
-
In your view, what would cause the cash book of an enterprise to disagree with the bank statement at the of month? Explain what you would include under Cost of sales of an enterprise during the...
-
Write a Lewis structure for the NO 3 ion. Include resonance structures.
-
The salinity of seawater can vary in the worlds oceans as shown in the map, which indicates salinity in units of percent by mass NaCl. Examine the image and answer the questions that follow. a. Which...
-
EnRG Inc. produces trail mix packaged for sale in convenience stores across Canada. At the beginning of April 2015, EnRG has no inventory of trail mix. Demand for the next three months is expected to...
-
What is the significance of the McCabe-Thiele method in the context of distillation design, and how does it compare to other graphical methods like the Fenske-Underwood-Gilliland (FUG) method?
-
In reactive distillation, how does the integration of chemical reactions within the distillation process affect equilibrium and kinetics? Discuss applications where this technique is particularly...
-
Discuss comprehensively the following competencies and practice behaviors that are expected during your field practicum : Engage in ongoing self-reflective practice including an awareness of and...
-
Tom, Jack and Harry paid a dividend of 120 cents. The dividends are expected to grow by 40% per annum during the 3-year supernormal period when the cost of equity is 30%, then grow at 20%per annum...
-
Bostian, Inc. has total assets of $540,000. Its total debt outstanding is $185,000. The Board of Directors has directed the CFO to move towards a debtto-assets ratio of 55%. How much debt must the...
-
Are all inventories included in current assets? Why or why not?
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Consider the plane electromagnetic wave in vacuum (in SI units) given by the expressions E x = 0, E y = 2 cos [2 10 14 (t - x/c) + /2], and E z = 0. (a) What are the frequency, wavelength, direction...
-
Write an expression for the vector E- and vector B-fields that constitute a plane harmonic wave traveling in the +z-direction. The wave is linearly polarized with its plane of vibration at 45 to the...
-
Considering Eq. (3.30), show that the expression is correct as it applies to a plane wave for which the direction of the electric field is constant. KxE= (3.30) Ey 3 .
-
As an Fire Officer, unforeseen challenges are going to constitute the bulk of your workdays. It might be something big, like a long-committed employee who decided to quit, or something small, like a...
-
Imagine a setting in which a group member declines to participate in a technique or exercise you suggest. Describe how you would respond. How could this refusal have different implications depending...
-
Imagine you have a small company at your home country (e.g. India, Philippine, Mexico), Choose a name for your company b. you want to act as an agent of a well-known Canadian company in one of the...
Applications Of Fluid Dynamics Proceedings Of ICAFD 2016 2016 Edition - ISBN: 9811053286 - Free Book

Study smarter with the SolutionInn App


